Epidemiology
RVF was first discovered in the Kenyan Rift Valley in the early 1910s in livestock, and the virus was identified in 1931.[4][5][11][12] Since then, outbreaks have occurred sporadically in sub-Saharan Africa, including in South Africa in 2010 and Mauritania in 2010, 2012, 2013 to 2014, 2015, and 2020.[13] In 2000 there was an outbreak in Saudi Arabia and Yemen, suggesting that spread to other parts of Asia and Europe is possible, but since this event there have been no human cases reported and few sporadic suspect livestock infections in this region. In 2016, outbreaks were reported in Niger (348 cases with 33 deaths) and in Uganda (3 confirmed and 2 probable cases); cases were detected after an apparent absence of Rift Valley fever virus (RVFV) over the previous 48 years. In July 2016, the first imported case was reported in China in a man returning from Angola.[14] An outbreak was reported in Kenya in June 2018 and November 2020, and a high number of deaths and abortions in livestock were reported, which was concerning given that people in the affected areas had reported consumption of meat from dead and sick animals.[15] The last outbreak was reported in Uganda between January and March 2023.[16]
In January 2019, five autochthonous cases of human infection were reported in Mayotte, a French archipelago located in the Indian Ocean off the coast of Southeast Africa between Mozambique and Madagascar. Between 22 November 2018 and 3 May 2019, 129 confirmed cases have been reported.[17] In October 2019, 47 suspected cases were reported in the Red Sea State in Sudan.[18] One systematic review of RVFV epidemiology over the past two decades confirmed that outbreaks are becoming more sporadic, frequent, and detected in new locations each year.[10] In fact, human cases have been detected yearly in East Africa since 2016 and there continues to be a lack of incidence data from the World Health Organization's One Health initiative to inform human risks. WHO: One Health Opens in new window The impact of climate change is expected to exacerbate arboviruses such as RVFV and possibly shift the burden away from malaria as arbovirus vectors tend to prefer warmer temperatures.[19]
Livestock and human infections are spatially correlated as viral amplification is thought to occur in these domestic ruminants before spillover to humans is initiated. Important for considering RVFV among differentials for non-specific fever, providers should not rely on confirmation of exposure to sick livestock in endemic areas as adult cattle (those primarily slaughtered and consumed) often have inapparent infections, and active surveillance in livestock is limited. Recognition of clinical signs in livestock is further complicated by the herd immunity effect and other common infections resembling RVF clinically. RVFV can also be transmitted by a wide range of arthropod vectors and exposure to these vectors is near ubiquitous in endemic regions. Nonetheless, human risk factors for RVFV confirmed by meta analysis include slaughtering, assisting livestock birth, disposing abortion tissues, sheltering livestock in the home, milking, and consumption of raw milk. However, there is a lack of endemic incidence data for RVFV and there is a confounded understanding of these risk factors because RVFV has primarily been studied in rural areas of Sub-Saharan Africa where more than 80% of people raise livestock at their homes. One study in urban areas of Kenya highlighted a potential risk for exposure via consumption of raw milk independent of livestock ownership.[20] There has never been a detected urban outbreak of RVFV driven by an urban transmission cycle, yet urban areas of endemic areas have all available hosts and vectors may have a preference for these environments. Once RVFV is introduced to a new area, it can persist via Aedesspp. mosquito vectors capable of vertical transmission laying infected eggs that can lay dormant for extended periods of time while waiting for flooding events. Thus, all areas that have previously detected RVFV could have ongoing low level transmission either in vectors, livestock, or even humans. Overall, RVFV epidemiology should be considered dynamic and risk for human transmission in a given location should be interpreted in the context of available diagnostics, ongoing active surveillance efforts, and recent data available on herd immunity in humans and livestock.
[Figure caption and citation for the preceding image starts]: Regional map of countries exposed to RVFV infection during the 1999-2021 era, based on findings of studies included in the systematic reviewGerken et al. 2022, PLOS Neglected Tropical Diseases, CC-BY 4.0; used with permission [Citation ends].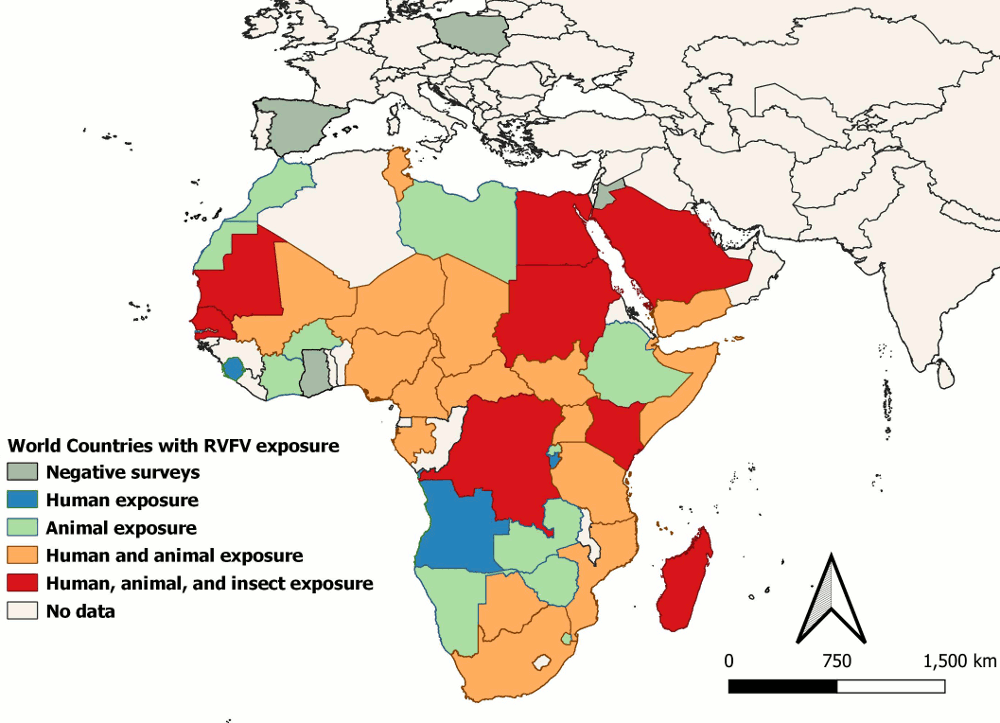
Use of this content is subject to our disclaimer