Recommendations
Urgent
Assessment and diagnosis of STEMI is a time-critical process. The shortest possible delay from symptom onset to coronary reperfusion maximises the patient’s chances of survival and recovery.[1][2][70][71][72][73][74][75]
Obtain an ECG immediately and certainly no more than 10 minutes from the point of first medical contact.[2][76][77]
Establish the patient’s haemodynamic status and specifically look for any signs of cardiogenic shock.
Cardiogenic shock complicates 5% to 10% of STEMI admissions. Look for persistent hypotension ( systolic blood pressure <90 mmHg) and/or any signs of end-organ hypoperfusion or fulminant heart failure.[78][79][80]
Make a clinical working diagnosis of STEMI based on a combination of acute chest pain (or equivalent symptoms suggestive of myocardial ischaemia) together with persistent ST-segment elevation in at least two contiguous ECG leads.[1][2]
As soon as a clinical diagnosis of STEMI is made, make an immediate assessment of eligibility for coronary reperfusion therapy (irrespective of age, ethnicity, sex, or level of consciousness) and seek input from the interventional cardiology team. Primary percutaneous coronary intervention is the preferred reperfusion strategy for any eligible patient who presents within 12 hours of symptom onset provided it can be delivered within 120 minutes of the time when fibrinolysis could have been given.[2][75][81]
Give all patients with suspected acute coronary syndrome a single loading dose of aspirin as soon as possible, unless they have aspirin hypersensitivity.[75]
Check your local protocol or discuss the patient with a senior colleague if they have hypersensitivity to aspirin.
Key Recommendations
Presentation
Patients typically present with central chest pain that is heavy in nature, like a sensation of pressure or squeezing. It may radiate to the left arm, neck, or jaw and can be associated with nausea, vomiting, dyspnoea, lightheadedness, palpitations, or syncope (chest pain-equivalent symptoms).[1][2][82]
Beware of presentations where chest pain is not the predominant feature (chest-pain equivalent symptoms), particularly in older patients, women, and patients with diabetes.[2][82]
Focus your history on:[2][71][74]
Characteristics of symptoms of myocardial ischaemia (including time since symptom onset, which will inform the most appropriate reperfusion strategy)
Previous cardiac history
Evidence of cardiac risk factors.
Examination is variable, and findings range from normal to a critically unwell patient in cardiogenic shock. Your priorities from examination and history-taking are to:[2][71][75][82]
Confirm a STEMI diagnosis
Rule out alternative diagnoses/causes of ST-segment elevation
Establish the patient’s haemodynamic status
Look for complications of acute MI.
Always consider right ventricular involvement when there is a triad of hypotension, elevated jugular venous pressure, and clear lung fields.[83]
Clinical diagnosis
In a patient who has chest pain or other ischaemic symptoms, make a clinical diagnosis of STEMI when there is new (or increased) and persistent ST-segment elevation in at least two contiguous leads of ≥1 mm in all leads other than leads V2-V3 where the following cut-off points apply:[1]
≥2.5 mm in men <40 years old
≥2 mm in men >40 years old
≥1.5 mm in women regardless of age
1 mm = 1 small square (at a standard ECG calibration of 10 mm/mV)
Contiguous ECG leads lie next to each other anatomically and indicate a specific myocardial territory.
Think posterior STEMI when there is deep ST-segment depression in leads V1-V3.[1][2]
Consider complete left main coronary artery obstruction if the following are both present, especially if the patient has haemodynamic compromise:[2][84]
ST depression ≥1 mm in ≥6 surface leads (i.e., inferolateral ST depression)
ST elevation in aVR or lead V1.
ECG diagnosis of STEMI is trickier in the presence of left bundle branch block (LBBB).
One of the best indicators is concordant ST-segment elevation (i.e., in leads with positive QRS deflections).[85] The Sgarbossa criteria can also be helpful.[1][2][86]
Manage any patient with bundle branch block and clinical suspicion of ongoing myocardial ischaemia as per the standard STEMI protocol. This applies to both left and right bundle branch block, whether new or previously known.[2]
Cardiac biomarkers
Although STEMI can usually be diagnosed by ECG alone, a rise in cardiac-specific troponins definitively confirms the diagnosis.[1][2][82][89][90][91][92]
However, do not delay coronary reperfusion to wait for cardiac biomarker laboratory results (or any other blood results).
Start treatment and assess eligibility for coronary reperfusion immediately a clinical diagnosis has been made based on ECG changes in a patient with symptoms/signs of myocardial ischaemia.
Practical diagnosis of STEMI
Assessment, diagnosis, and management of acute STEMI is a time-critical process.[1][2][71][74][75]
Always remember the guiding principle that 'time is muscle' – the shortest possible delay from symptom onset to coronary reperfusion is vital to protect the myocardium from ischaemic damage and maximise the patient’s chances of survival.[72][73]
For a patient diagnosed with STEMI, nearly half of potentially salvageable myocardium is lost within 1 hour of the coronary artery occlusion and two-thirds is lost within 3 hours.[93]
Make a working clinical diagnosis of STEMI and start immediate treatment if the patient meets BOTH of the following criteria:[2]
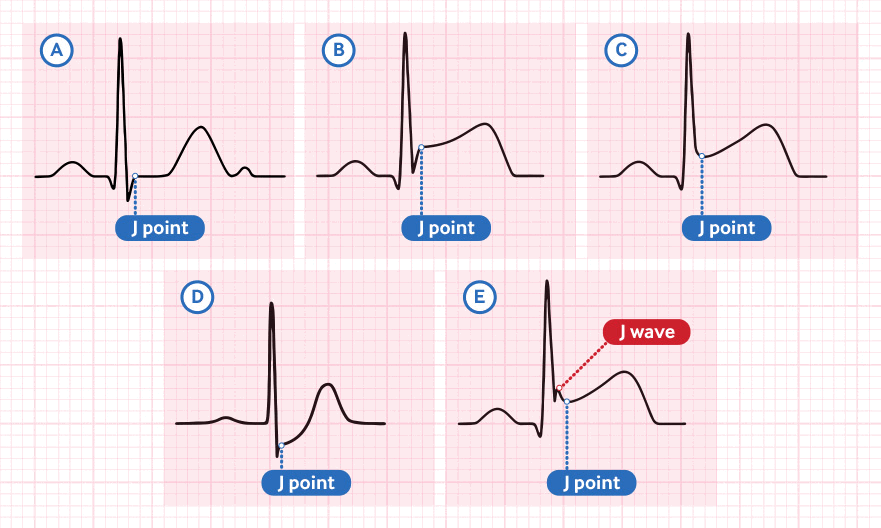
New ST-segment elevation at the J-point in at least two contiguous leads on a 12-lead ECG of ≥1 mm in all leads (in the absence of left ventricular hypertrophy or left bundle branch block) other than leads V2-V3 where the following cut-off points apply:[1][2]
≥2.5 mm in men <40 years old
≥2 mm in men >40 years old.
Persistent typical central chest pain or other symptoms consistent with myocardial ischaemia (chest pain-equivalent symptoms) within the last 12 hours.
As soon as a clinical diagnosis is made, immediately assess eligibility for coronary reperfusion therapy.[2][75]
Do not wait for a definitive diagnosis from cardiac troponin levels as coronary reperfusion is a time-critical intervention.[1][2]
Primary percutaneous coronary intervention (PCI) is the preferred reperfusion strategy for any eligible patient who presents within 12 hours of symptom onset provided it can be delivered within 120 minutes of the time when fibrinolysis could have been given.[75][81] Start medical treatment and refer immediately to the interventional cardiology team.[2][72][73][75]
Give fibrinolysis (unless contraindicated) if primary PCI cannot be delivered within 120 minutes of the time when fibrinolysis could be started.[75][81]
Practical tip
The European Society of Cardiology recommends to 'Think A.C.S.' at initial assessment of chest pain:[2]
Abnormalities or evidence of ischaemia on ECG assessment
Clinical context: take a targeted clinical history to assess the clinical context of the presentation
Stability: targeted clinical examination to assess for clinical and haemodynamic stability.
The admitting team can decide whether immediate invasive management is required based on this initial assessment.
Formal diagnostic criteria for STEMI
A definitive diagnosis of STEMI requires evidence of a rise in cardiac troponin levels – but do not wait for this to be confirmed before starting treatment.[1]
Evidence of myocardial injury (via acutely elevated cardiac troponin levels) is required for a definitive confirmation of the diagnosis of STEMI.[1][2][82]
However, coronary reperfusion is a time-critical intervention that must be started as soon as a clinical diagnosis is made.[2][75]
STEMI is classified as a type 1 MI under the Fourth Universal Definition of Myocardial Infarction.[1]
Type 1 MIs are caused by ischaemia due to rupture or erosion of an atherosclerotic plaque leading to partial, or in the case of STEMI total, intraluminal occlusion of the coronary artery.[1]
Under the Fourth Universal Definition, an acute type 1 MI is definitively diagnosed based on a rise and/or fall of cardiac troponin levels, with at least one value above the 99th percentile of the upper reference limit in a patient who also has at least one of the following:[1][82]
Symptoms of acute myocardial ischaemia
ECG changes indicative of new ischaemia
Development of pathological Q waves in the ECG
A new regional left ventricular wall motion abnormality consistent with a coronary artery territory (e.g., on transthoracic echocardiography)
New loss of viable myocardium (e.g., on cardiac magnetic resonance imaging)
Presence of intracoronary thrombus found on coronary angiography.
The presenting ECG is central to determining whether a type 1 MI is a STEMI or a non-ST-elevation MI (NSTEMI).[1]
Patients typically present with central chest pain:[1][2][82]
Classically retrosternal, crushing, heavy, severe and diffuse in nature
Might be described by the patient as 'pressing or squeezing'
May occur at rest or on activity
May be constant or intermittent, or wax and wane in intensity
Sometimes radiating to the left arm, neck, or jaw.
The chest pain may be associated with nausea, vomiting, dyspnoea, diaphoresis, lightheadedness, palpitations, or syncope.[2][82]
Dyspnoea is a common feature secondary to pulmonary congestion from left ventricular systolic dysfunction. It can also occur due to other mechanical and electrical complications of acute MI, which occur less commonly in the context of contemporary rapid revascularisation, for example:
Left ventricular aneurysm
Ventricular septal rupture
Left ventricular free wall rupture
Acute mitral regurgitation – papillary muscle rupture/functional (ischaemic) mitral regurgitation
Pericardial effusion
Cardiac tamponade
Supraventricular tachyarrhythmias
Ventricular tachyarrhythmias
Bradycardia and atrioventricular block.
Nausea and vomiting are common features.
These are non-specific symptoms but are commonly associated with inferior-wall STEMI due to increased vagal tone.
May be the only indicator of inferior-wall STEMI.
Patients sometimes report anxiety and/or an impending sense of doom.
Some patients present with palpitations.[2][94]
Tachycardia
Supraventricular tachyarrhythmias such as atrial fibrillation
Ventricular tachyarrhythmias such as ventricular tachycardia
Bradycardia
Sinus bradycardia
Atrioventricular block secondary to inferior STEMI
Atrioventricular block secondary to anterior STEMI
Irregular heart beat
Supraventricular tachyarrhythmias such as atrial fibrillation
Ventricular extrasystoles
Be aware of patients who present without chest pain as the predominant feature (i.e., with chest pain-equivalent symptoms).[2][82]
Women, older patients, and patients with diabetes are more likely to present with such features.[2][95]
Patients might describe their chest symptoms as burning, throbbing, tight or a feeling like trapped wind.
The patient may describe indigestion rather than chest pain.
In the absence of chest pain, there may be epigastric pain, back (interscapular) pain, neck or jaw pain, or arm pain (typically left-sided).
Patients may present with breathlessness, sweating, palpitations, dizziness, nausea, or vomiting but no chest pain.
Clinical suspicion is key to making the diagnosis. It is, therefore, vital to make a full assessment based on the history, examination, and serial ECGs.[1][92]
Practical tip
Do not rely on a positive patient response to glyceryl trinitrate as a reliable diagnostic indicator of ischaemic chest pain.[2][82][96]
Response to nitrates can be misleading. Patients who get symptom relief still need confirmatory ECG testing to inform the diagnosis.
Complete normalisation of ST-segment elevation along with resolution of chest pain after buccal or sublingual nitrates suggests coronary vasospasm (with or without associated MI).[2]
Patients with STEMI may be asymptomatic – this is known as a silent STEMI.[1]
STEMI is rarely truly asymptomatic but some patients have only mild, non-specific symptoms that can lead to a delay in presenting or to the STEMI going undiagnosed.
In practice, a patient who has a 'silent' STEMI may present to their primary care doctor a few days after the episode of non-specific symptoms, at which point evidence of ST-segment elevation might still be present on the ECG. Seek advice from the cardiology team for such patients.
Your history should cover the following.[82][97]
Characteristics of symptoms; in particular, chest pain:[82]
“Have you ever had this type of pain before?”
Nature, severity, and duration of pain
Radiation
Associated symptoms
“Do you normally have chest pain when you exert yourself? If so, is it similar in quality to the chest pain experienced when you don’t?”
Time since symptom onset – this is crucial to inform the appropriate reperfusion strategy.[2][74][75]
If symptoms are intermittent, it is important to ask when the last episode of pain occurred.[82]
Any history of cardiovascular disease; in particular, ischaemic heart disease.[82]
Also check for any previous episodes of investigation or treatment for chest pain.
Cardiovascular risk factor profile:[82]
Smoking status
Hypertension
Diabetes mellitus
Hypercholesterolaemia
Family history of premature coronary artery disease (<60 years)
Established coronary artery disease
Advanced age
Obesity
Metabolic syndrome
Physical inactivity
Chronic kidney disease
Cocaine use
Existing peripheral vascular disease or cerebrovascular disease.
Medication history:
Will help to consolidate the risk factor profile assessment, especially if the history given by the patient is limited or vague
Record any use of chronic oral anticoagulation – this will influence what type of arterial access can be used if the patient proceeds to coronary angiography or primary percutaneous coronary intervention (PCI).
Practical tip
The choice of coronary reperfusion strategy depends on time since symptom onset – but obtaining an exact time for this can be difficult.
Patients can often give only an approximate idea of when their symptoms began.
Patients sometimes ignore chest pain (or associated symptoms) until they can no longer tolerate it.
The reliability of the assessment of time since symptom onset is determined by a combination of the patient’s ability to give an accurate history and the experience and skill of the clinician taking the history.
If you question the patient carefully, they may describe warning signs, or less severe or less long-lasting symptom episodes preceding the more severe episode that has prompted them to seek medical help.
The total ischaemic time encompasses time from symptom onset until coronary reperfusion with either primary PCI or fibrinolysis.
If the patient contacts emergency medical services in the community, then the total ischaemic time = Patient Delay + Emergency Medical Services Delay + System Delay.
If the patient presents directly to a hospital (PCI-capable or non-PCI-capable), then the total ischaemic time = Patient Delay + System Delay.
The clinical picture of acute MI varies from asymptomatic through to fulminant acute heart failure and cardiogenic shock.
Your priorities are to establish the following as quickly as possible:[82]
Confirm the STEMI diagnosis
Rule out alternative diagnoses/causes of ST-segment elevation (see Important differentials to consider below)
Establish the patient’s haemodynamic status – seek immediate senior support and specialist input if there are any signs of cardiogenic shock (see Cardiogenic shock below)
Look for complications of acute MI (see Acute MI complications sections below).
Your examination should check:[82]
Blood pressure, heart rate, and heart rhythm
Consciousness level (e.g., Glasgow Coma Scale, AVPU [alert, verbal, pain, unresponsive] scale)
However, do not use the level of consciousness after cardiac arrest caused by suspected acute STEMI to determine whether the patient is eligible for coronary angiography ± primary percutaneous coronary intervention (PCI)[75]
Airway patency
Oxygen saturations
Pulse: radio-radial and radio-femoral delay
Jugular venous pressure (JVP) – a raised JVP could indicate:
Congestive cardiac failure
Right ventricular involvement after an inferior or extensive anterior STEMI
Underlying (chronic) lung disease
Large pericardial effusion or cardiac tamponade
Pulmonary embolism (which can also give rise to ST-segment elevation).
Look for pallor, cool/clammy to touch skin, and any signs of peripheral shutdown.
These are common presenting features due to high sympathetic output resulting in peripheral vasoconstriction.
Auscultate the heart and lungs.
Muffled heart sounds could suggest a pericardial effusion or even cardiac tamponade.
Is there a third (S3) or fourth (S4) heart sound?
These added heart sounds could suggest severe heart failure.
A murmur might suggest:
Acute ventricular septal defect
Acute mitral regurgitation
Underlying chronic valvular heart disease.
Crackles/crepitations or cardiac wheeze would suggest congestive cardiac failure ± pulmonary oedema.
Is the patient coughing up pink frothy sputum?
This would suggest congestive cardiac failure ± pulmonary oedema.
Check for peripheral oedema and hepatomegaly.
Document the Killip class. This classifies degree of heart failure after acute MI and predicts 30-day mortality:[97]
Killip class I = no signs of heart failure/pulmonary congestion
Killip class II = S3 and basal crackles/crepitations
Killip class III = acute pulmonary oedema
Killip class IV = cardiogenic shock.
Evidence: Killip class and prognosis
Risk of death is strongly correlated to Killip class.
The original paper on the impact of Killip class on prognosis dates from 1967, when it was reported that the associated in-hospital mortality rates were 6% for class I, 17% for class II, 38% for class III, and 81% for class IV.[98]
With advances in treatment, particularly the introduction of coronary reperfusion therapy, mortality rates have fallen by 30% to 50% in each Killip class. In the GUSTO international trial of 41,021 patients, after adjustment for all other factors, the OR associated with Killip class III versus I for an average-age patient was 4.37 (95% CI, 3.34 to 5.71), whereas the OR for Killip class IV versus I was 7.86 (95% CI, 5.88 to 10.49).[97]
Cardiogenic shock
Cardiogenic shock complicates 5% to 10% of STEMI admissions.[79][80][99][100]
In-hospital mortality remains high (≥50%).[100]
There is a bimodal presentation: the majority occur within 24 hours; the remainder occur within the first week.[100][101]
Seek immediate senior support and specialist input if your clinical assessment suggests cardiogenic shock.[79]
See Shock.
Patients present with signs of hypoperfusion and/or fulminant heart failure, such as:
Altered mental status/reduced consciousness
Tachypnoea
Severe dyspnoea
Tachycardia
Orthopnoea
Cool peripheries
Grey, ashen, pale appearance.
Cardiogenic shock is defined as persistent hypotension (systolic blood pressure [SBP] <90 mmHg) together with signs of end-organ hypoperfusion.[78][79][80]
SBP <90 mmHg despite adequate volume replacement, or if inotropes and/or mechanical circulatory support are needed to maintain SBP ≥90 mmHg
Urine output <30 mL/hour
Cool extremities.
Haemodynamic criteria:[78][80]
Cardiac index ≤2.2 L/minute/m2
Wedge pressure ≥15 mmHg.
Cardiogenic shock results from extensive left ventricular infarction and/or mechanical complications such as:[80]
Papillary muscle rupture
Ventricular septal rupture
Left ventricular free wall rupture leading to pericardial tamponade
Right ventricular infarction.
Important differentials to consider
Always consider alternative diagnoses that might explain the presenting symptoms and/or ST elevation on ECG, including:[82]
Aortic dissection (ST elevation can be present on the ECG)
Pulmonary embolism (ST elevation or ST depression can be present on the ECG)
Pericarditis (ST elevation can be present on the ECG)
Myocarditis (ST elevation can be present on the ECG)
Pneumothorax
Pneumonia
Intracranial pathology (e.g., subarachnoid haemorrhage)
Gastro-oesophageal reflux disease
Oesophageal spasm
Costochondritis
Anxiety or panic.
Be aware of spontaneous coronary artery dissection (SCAD), especially in younger women presenting with ST-segment elevation and chest pain.
SCAD is defined as an epicardial coronary artery dissection that is not associated with atherosclerosis or trauma and is not iatrogenic.[102][103]
The left anterior descending artery is the most commonly affected artery.[102][104][105][106][107][108][109]
It can lead to coronary artery obstruction secondary to intramural haematoma or intimal disruption (in contrast to the atherosclerotic plaque rupture or intraluminal thrombus seen with STEMI).
Patients almost always present with an acute coronary syndrome causing chest pain and elevation in cardiac enzymes.[102]
Consider the possibility of SCAD in any young patient (especially female) who presents with an acute MI and who has no history of or risk factors for cardiovascular disease.[102]
SCAD is an important cause of ST-segment deviation on the ECG, with different patient series reporting that:[102][104][105][106][107][108]
26% to 87% of patients with SCAD present with STEMI
13% to 69% present with non-STEMI (NSTEMI).
If SCAD is suspected, coronary angiography should be performed as soon as possible to confirm the diagnosis.[102][103]
There is a paucity of evidence available to guide management decisions once SCAD is confirmed on angiography. The most appropriate approach depends on individual patient characteristics.
Conservative management with close ongoing monitoring is preferred for most patients who are clinically stable, as observational data suggest the SCAD lesion will usually heal.[102][103]
Urgent intervention with PCI or coronary artery bypass grafting should be considered for high-risk patients (e.g., ongoing ischaemia, haemodynamic instability, left main artery dissection).[112]
Practical tip
Takotsubo cardiomyopathy syndrome can mimic MI and has similar mortality to STEMI/NSTEMI.[1]
It is triggered by a wide spectrum of physical and emotional triggers and is also referred to in the literature as broken heart syndrome, apical ballooning cardiomyopathy, or stress cardiomyopathy.[113][114][115]
'Takotsubo' comes from the Japanese word for octopus trap.
Takotsubo cardiomyopathy is characterised by a temporary left ventricular wall motion abnormality associated with signs and symptoms of acute coronary syndrome.[114][115] For example:
Chest pain
ST-segment deviation on ECG
Raised cardiac biomarkers such as troponin (although the peak value is often modest).[1]
In-hospital mortality is similar to STEMI and NSTEMI.[1]
It is estimated to represent 1% to 3% of all patients and 5% to 6% of female patients who present with suspected STEMI.[114] Over 90% of affected patients are post-menopausal women.[1]
Patients may present with ST-segment elevation on their ECG.[114][115]
ST-segment elevation is present in over 40% of patients but the extent of the elevation is usually widespread across the lateral and precordial leads, beyond that of a single coronary artery distribution.[1]
Suspect takotsubo cardiomyopathy if the clinical manifestations and ECG abnormalities are out of proportion to the degree of elevation of cardiac biomarkers;[1] echocardiography findings may include hyperdynamic basal segments with apical ballooning.
Manage the patient as for STEMI in the first instance if there are signs and symptoms consistent with myocardial ischaemia.
Refer for urgent coronary angiography and left ventriculography to confirm or exclude takotsubo cardiomyopathy.
If there are coronary culprit lesions on angiography that correspond to the regional wall motion abnormalities, the patient is treated the same as for an acute coronary syndrome.
If there are no coronary culprit lesions that correspond to the regional wall motion abnormalities and acute infectious myocarditis can be ruled out, the patient is treated as a takotsubo cardiomyopathy.
Co-existing coronary artery disease does not exclude the diagnosis.
The distinction between MI and takotsubo cardiomyopathy syndrome can be made by the presence of QTc prolongation >500 ms during the acute phase and the recovery of left ventricular function over 2-4 weeks.[1]
ECG interpretation for STEMI
Perform a 12-lead ECG within 10 minutes of first medical contact in any patient who presents with chest pain and/or other signs of possible STEMI.[1][2]
If the patient presents in the community, obtain a pre-hospital ECG and send it digitally to the receiving hospital as quickly as possible.[2][82][119]
If the ECG is equivocal despite a high clinical suspicion of acute MI, perform serial ECGs in the appropriate hospital setting and compare these with historical ECGs, if available.[1][2][82]
In the appropriate clinical context (chest pain or other symptoms of ischaemia), make a clinical working diagnosis of STEMI if there is new (or increased) and persistent ST-segment elevation in two or more contiguous leads of ≥1 mm in all leads other than leads V2-V3 where the following cut points apply:[1]
≥2.5 mm in men <40 years old
≥2 mm in men >40 years old
≥1.5 mm in women regardless of age.
1 mm = 1 small square (at a standard ECG calibration of 10 mm/mV).
Make the diagnosis when these criteria are met in the absence of left ventricular hypertrophy, left bundle branch block (LBBB), or a paced rhythm on the ECG.
Severe cases of left ventricular hypertrophy can appear identical to LBBB.
A paced rhythm can appear identical to LBBB.
Note that the presence of left ventricular hypertrophy, LBBB, or a paced rhythm does not preclude a diagnosis of STEMI if the patient presents with typical symptoms of myocardial ischaemia.
See Less common ECG presentations below for more on diagnosis of STEMI in the presence of LBBB.
Consult a cardiology specialist immediately if the ECG changes are equivocal.
An urgent transthoracic echocardiogram to look for regional wall motion abnormalities is indicated.[120]
Practical tip
Contiguous leads lie next to each other anatomically and indicate a specific myocardial territory. [Figure caption and citation for the preceding image starts]: 12-lead ECG placementCreated by Npatchett (own work) [CC BY-SA 4.0], via Wikimedia Commons [Citation ends].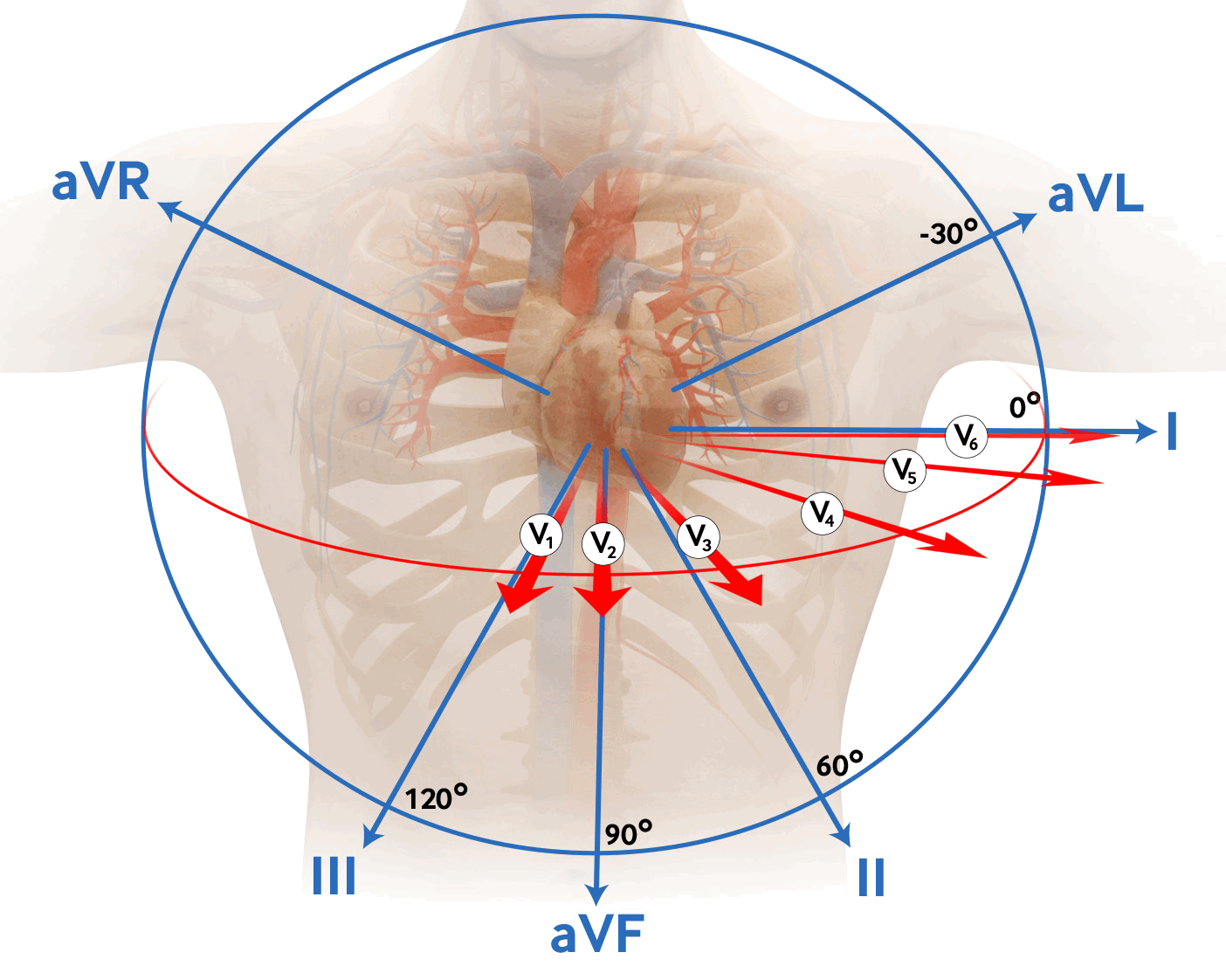 [Figure caption and citation for the preceding image starts]: Coronary anatomy and ECG leadsCreated by the BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Coronary anatomy and ECG leadsCreated by the BMJ Knowledge Centre [Citation ends].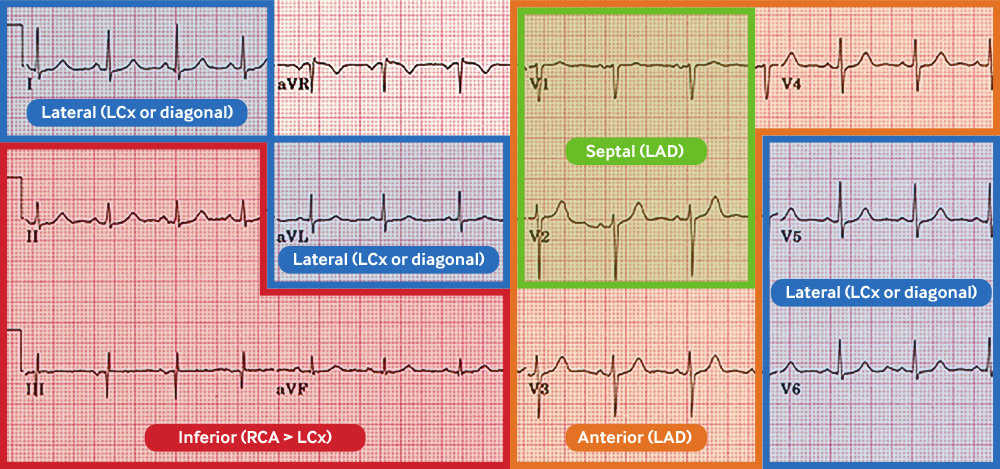 [Figure caption and citation for the preceding image starts]: Coronary anatomy and ECG leads tableCreated by the BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Coronary anatomy and ECG leads tableCreated by the BMJ Knowledge Centre [Citation ends].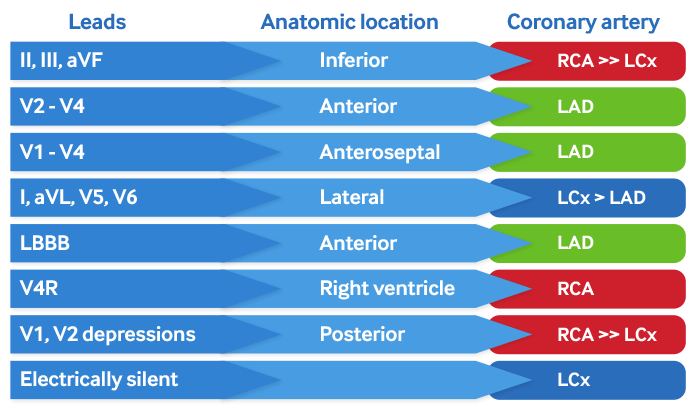
Less common ECG presentations
Posterior STEMI[1][2]
Consider this when there is ST-segment depression in leads V1-V3 along with characteristic signs and symptoms of myocardial ischaemia.
Confirm with posterior lead ECG: ST-segment elevation ≥0.5 mm in V7-V9.
Right ventricular infarction[1][2][121]
Can complicate an inferior STEMI.
Check right precordial leads (V3R and V4R) for ST-segment elevation.
Look for ST elevation ≥1 mm in aVR and V1.
Confirmation will have an impact on choice of therapeutic intervention.
Left main coronary obstruction[2][84]
Consider complete left main coronary artery obstruction if the following are both present, especially if the patient has haemodynamic compromise:
ST depression ≥1 mm in ≥6 surface leads (i.e., inferolateral ST depression)
ST elevation in aVR or lead V1.
STEMI in the presence of LBBB
ECG diagnosis of STEMI is trickier in the presence of LBBB.[2][86][122]
Bundle branch block (BBB) precludes accurate assessment, but it may be possible to make the diagnosis if marked ST-segment abnormalities are present.[2]
The presence of concordant ST-segment elevation (i.e., in leads with positive QRS deflections) is one of the best indicators of total coronary occlusion and ongoing MI in the context of a patient with concomitant LBBB.[85]
Manage any patient with BBB and clinical suspicion of ongoing myocardial ischaemia as per the standard STEMI protocol. This applies to both left and right bundle branch block, whether new or previously known.[2]
Presumed new LBBB alone does not indicate the presence of a STEMI.[87][88]
If in doubt, seek immediate input from the cardiology team.
More info: Sgarbossa criteria for diagnosis of MI in the presence of LBBB
Consider using the Sgarbossa criteria to improve the diagnostic accuracy for STEMI in patients who have LBBB at presentation.
ST elevation of ≥1 mm, concordant with the QRS complex: 5 points.
ST depression ≥1 mm in leads V1, V2, or V3: 3 points.
This has a sensitivity of 19% and a specificity of 81% to diagnose acute MI.[123]
ST elevation ≥5 mm, discordant with the QRS complex: 2 points.
This has a sensitivity of 10% and a specificity of 99% to diagnose acute MI.[123]
An aggregated score of 3+ is 90% specific for MI but only 36% sensitive.
Components of the Sgarbossa criteria have high specificity but low sensitivity so are useful to confirm acute MI but less useful to rule it out.[124]
The low sensitivity means you must maintain a high index of suspicion if the presentation is consistent with MI regardless of the criteria score.
'Weighted' Sgarbossa criteria rely on the points system; however, only two of the criteria carry a score ≥3 to make the diagnosis of acute MI.[125]
'Unweighted' Sgarbossa criteria are applied without the points system – this is more sensitive but less specific.[125]
The criteria were originally based on the outcome of acute MI as measured by creatine kinase-MB rather than angiographic evidence of acute coronary occlusion – further reducing sensitivity because the criteria encompass both STEMI and NSTEMI.[125]
[Figure caption and citation for the preceding image starts]: Sgarbossa criteria for MI in the presence of LBBBCreated by the BMJ Knowledge Centre [Citation ends].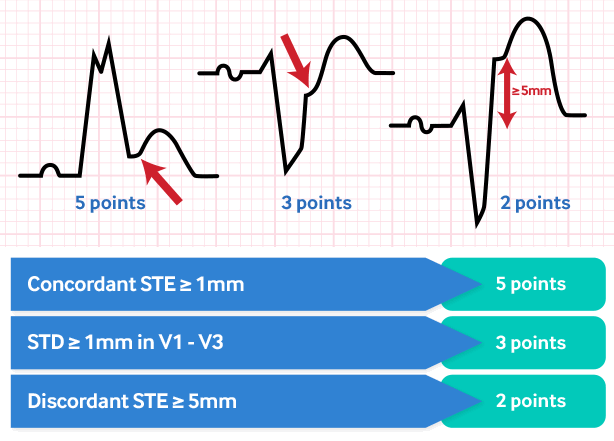
The modified Sgarbossa criteria have better sensitivity but worse specificity for STEMI.[125][126]
The original rule for >5 mm discordance is replaced with a proportionately excessive discordance: ST-elevation/S-wave amplitude ≤-0.25.
The modified criteria were found to be more sensitive versus the 'weighted' (80% vs. 49%; P <0.001) and 'unweighted' (80% vs. 56%; P <0.001) Sgarbossa criteria.[125]
[Figure caption and citation for the preceding image starts]: ST/S ratio under the modified Sgarbossa criteriaCreated by the BMJ Knowledge Centre [Citation ends].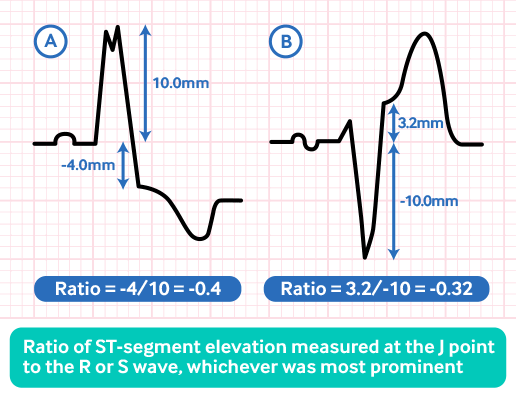
Previous silent/unrecognised STEMI
Be aware of the possibility that abnormal ECG features might be due to a previous silent/unrecognised STEMI.[1]
Residual ST elevation on the ECG from an old STEMI may be detected either incidentally in an asymptomatic patient who is having an ECG for another reason, or occasionally in a patient with symptoms of ischaemia who is experiencing a non-ST-elevation MI (NSTEMI) against the background of a previous history of STEMI.
In such cases, there may be ST-segment elevation on the current ECG that was already present on old ECGs (e.g., in the case of left ventricular aneurysm formation). It is important to distinguish this from new ST elevation as management will differ.
The historical STEMI may have been 'silent' and gone unrecognised at the time. The resulting old ECG features will usually be fixed.
It is helpful to take a thorough history, together with diligent ECG interpretation and comparison with old ECGs (plus medical records) if available.
Seek specialist input from the cardiology team.
The following criteria for previous (or silent/unrecognised) MI can be helpful. There may be:[1]
Pathological Q waves with or without symptoms, in the absence of non-ischaemic causes[1]
Loss of viable myocardium in a pattern consistent with a coronary artery territory (regional wall abnormality) seen on cardiac imaging:
Echocardiography
Myocardial perfusion scintigraphy (MPS)
Positron emission tomography (PET)
Cardiac magnetic resonance imaging
Pathological findings of a healed or healing MI on cardiac imaging.
Practical tip
A Q wave is defined as any negative deflection preceding an R wave in the QRS complex.
A Q wave represents the normal left-to-right depolarisation across the interventricular septum.
Small Q waves tend to be normal in most leads (e.g., left-sided leads such as I, aVL, V5, and V6).
More pronounced Q waves (>2 mm deep) may be seen in lead III or aVR as a normal variant.
Q waves are not usually seen in the right-sided leads (V1-V3).
Pathological Q waves can be a sign of previous MI. The precise definition of pathological Q waves has been debated. The 2018 Fourth Universal Definition of MI defines them as follows:[1]
Any Q wave in leads V2-V3 >20 milliseconds (0.02 seconds) or any QS complex in leads V2 and V3
Q wave ≥30 milliseconds (0.03 seconds) and ≥1 mm deep or QS complex in leads I, II, aVL, aVF, or V4-V6 in any two leads of a contiguous lead grouping (I, aVL; V1-V6; II, III, aVF)
R wave >40 milliseconds (0.04 seconds) in V1-V2 and R/S >1 with a concordant positive T wave in the absence of a conduction defect.
ECG examples
Anterior STEMI
[Figure caption and citation for the preceding image starts]: Anterior STEMIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].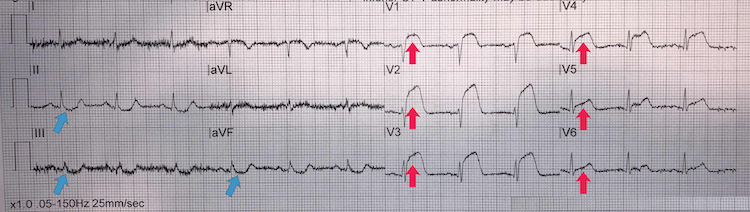 Key learning points
Key learning points
Tombstone’ ST elevation in anterior chest leads V1-V6 = anterior STEMI (red arrows)
Reciprocal inferior ST-segment depression in II, III, and aVF (blue arrows)
This is a high-risk ECG
Anterolateral STEMI example I
[Figure caption and citation for the preceding image starts]: Anterolateral STEMI example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].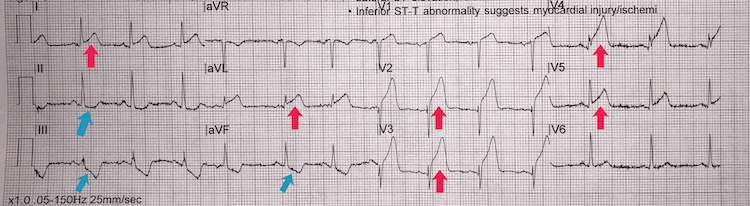 Key learning points
Key learning points
‘Tombstone’ ST elevation in leads V2-V6, I, and aVL = anterolateral STEMI (red arrows)
Reciprocal inferior ST-segment depression in II, III, and aVF (blue arrows)
High lateral STEMI
[Figure caption and citation for the preceding image starts]: High lateral STEMIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].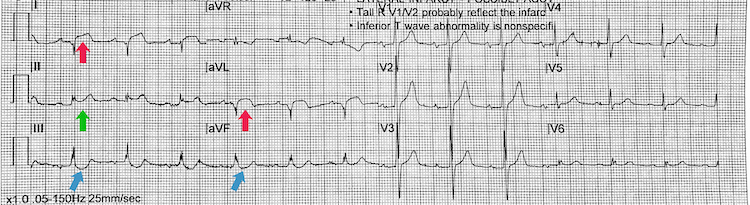 Key learning points
Key learning points
ST-segment elevation in high lateral chest leads I and aVL = high lateral STEMI (red arrows)
Reciprocal inferior ST-segment depression in leads III and aVF (blue arrows)
There is also saddle-shaped ST-segment elevation in lead II (green arrow) – difficult to state the significance of this
Inferoposterior STEMI example II
[Figure caption and citation for the preceding image starts]: Inferoposterior STEMI example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].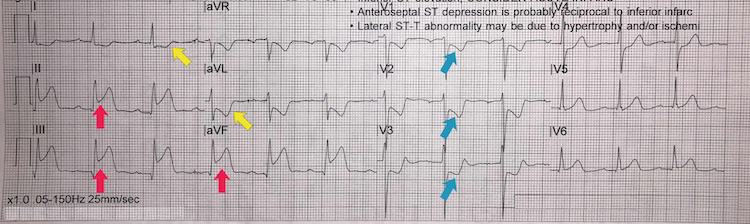 Key learning points
Key learning points
Note the deep ST-segment depression and R:S wave ratio of >1 in V1-V3 = posterior STEMI (blue arrows)
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
Reciprocal ST-segment depression in the lateral leads I and aVL (yellow arrows)
Consider performing a posterior lead ECG (leads V7-V9) for further confirmation of a posterior STEMI
Inferoposterolateral STEMI example III
[Figure caption and citation for the preceding image starts]: Inferoposterolateral STEMI example IIIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].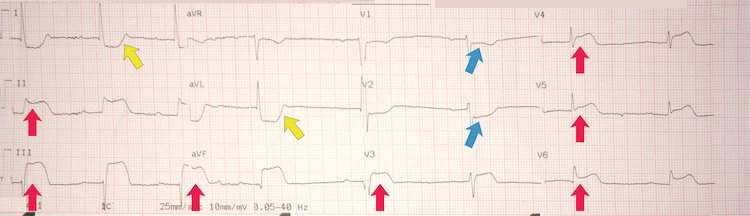 Key learning points
Key learning points
Note the deep ST-segment depression and R:S wave ratio of >1 in V1-V2 = posterior STEMI (blue arrows)
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
ST-segment elevation in leads V3-V6 = lateral STEMI (green arrows)
Reciprocal deep ST-segment depression in leads I and aVL (yellow arrows)
The patient has marked sinus bradycardia – there can be several reasons for this but with this degree of ischaemia on the ECG it suggests current or impending haemodynamic instability
More info: Library of ECGs with learning points
Anterolateral STEMI example II
[Figure caption and citation for the preceding image starts]: Anterolateral STEMI example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends]. Key learning points
Key learning points
ST elevation in leads V2-V5, I, and aVL = anterolateral STEMI (red arrows)
Reciprocal inferior ST-segment depression in II, III, and aVF (blue arrows)
Note there are no pathological Q waves in the anterior chest leads (V2-V6) – contrast with Anterolateral STEMI example I)
Anteroseptal STEMI example I
[Figure caption and citation for the preceding image starts]: Anteroseptal STEMI example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].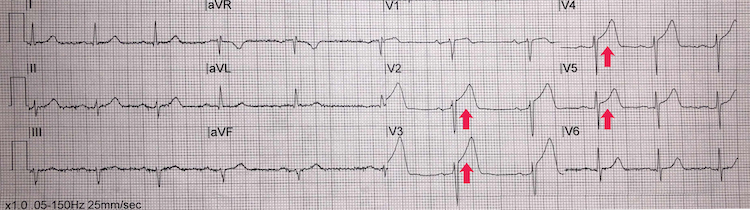 Key learning points
Key learning points
ST-segment elevation in leads V2-V5 = anteroseptal STEMI (red arrows)
There are, however, no reciprocal ischaemic changes on the ECG
Reciprocal ST-segment depression represents either true distant ischaemia as a by-product of a collateral circulation or an electrical phenomenon arising from a mirror reflection of ST-segment elevation elsewhere
Take a careful history and examination with this ECG – the anteroseptal ST-segment elevation may represent ‘high take-off’(or early benign repolarisation) rather than true myocardial ischaemia
If there are signs and symptoms of ongoing myocardial ischaemia then treat as a STEMI
Anteroseptal STEMI example II
[Figure caption and citation for the preceding image starts]: Anteroseptal STEMI example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].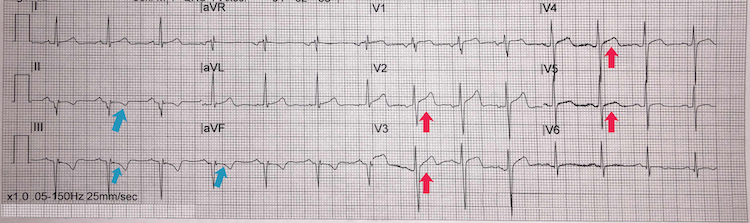 Key learning points
Key learning points
ST-segment elevation in leads V2-V5 = anteroseptal STEMI (red arrows)
Reciprocal ST-segment depression in the inferior leads II, III, and aVF
Compared with Anteroseptal STEMI example I: here the ST-segment elevation is less pronounced but the reciprocal ST-segment depression inferiorly make this ECG more compelling for an acute MI
Again, take a careful history and examination looking for signs and symptoms of ongoing myocardial ischaemia
Left bundle branch block example I
[Figure caption and citation for the preceding image starts]: Left bundle branch block example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].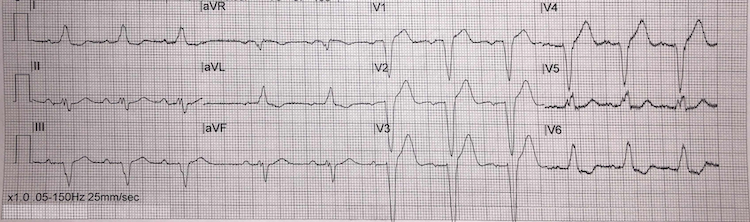 Key learning points
Key learning points
Left bundle branch block (LBBB) ECG criteria:
QRS duration >120 milliseconds (i.e., greater than 3 small squares on the ECG)
Monomorphic R wave in leads I, V5, and V6 – look for the characteristic ‘M-pattern’ shape (notched R wave) to the QRS complex in leads V5 and V6
Deep and broad S wave in leads V1-V2
ST-segment depression and T-wave inversion in left-sided leads (V5, V6, I, and aVL)
ST-segment elevation and positive T waves in V1-V3 (ST-segment elevation rarely exceeds >5 mm – refer to Sgarbossa versus modified Sgarbossa criteria)
Left bundle branch block example II
[Figure caption and citation for the preceding image starts]: Left bundle branch block example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].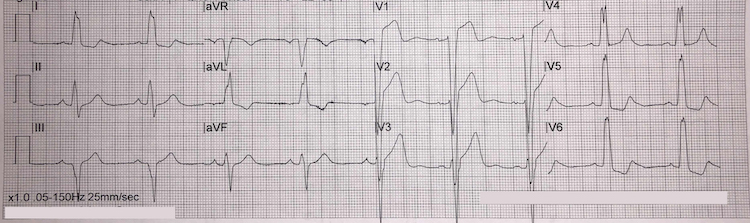 Key learning points
Key learning points
LBBB ECG criteria:
QRS duration >120 milliseconds (i.e., greater than 3 small squares on the ECG)
Monomorphic R wave in leads I, V5, and V6 – look for the characteristic ‘M-pattern’ shape (notched R wave) to the QRS complex in leads V5 and V6
Deep and broad S wave in leads V1-V2 – more pronounced when compared with example I
ST-segment depression and T-wave inversion in left-sided leads (V5, V6, I, and aVL)
ST-segment elevation and positive T waves in V1-V3 (ST-segment elevation rarely exceeds >5 mm – refer to Sgarbossa versus modified Sgarbossa criteria)
Left bundle branch block example III
[Figure caption and citation for the preceding image starts]: Left bundle branch block example IIIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].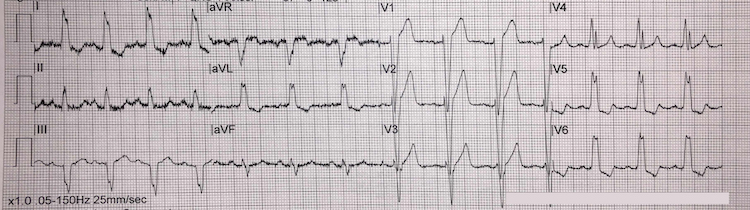 Key learning points
Key learning points
LBBB ECG criteria:
QRS duration >120 milliseconds (i.e., greater than 3 small squares on the ECG)
Monomorphic R wave in leads I, V5, and V6 – look for the characteristic ‘M-pattern’ shape (notched R wave) to the QRS complex in leads V5 and V6, which are very pronounced here
Deep and broad S wave in leads V1-V2 – just as pronounced when compared with example II
ST-segment depression and T-wave inversion in left-sided leads (V5, V6, I, and aVL)
ST-segment elevation and positive T waves in V1-V3 (ST-segment elevation rarely exceeds >5 mm – refer to Sgarbossa versus modified Sgarbossa criteria)
Inferoposterior STEMI example I
[Figure caption and citation for the preceding image starts]: Inferoposterior STEMI example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].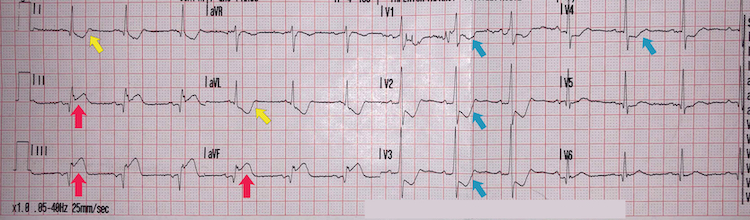 Key learning points
Key learning points
Note the deep ST-segment depression and R:S wave ratio of >1 in V1-V4 = posterior STEMI (blue arrows)
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
Reciprocal ST-segment depression in the lateral leads I and aVL (yellow arrows)
Consider performing a posterior lead ECG (leads V7-V9) for further confirmation of a posterior STEMI
Inferoposterolateral STEMI example I
[Figure caption and citation for the preceding image starts]: Inferoposterolateral STEMI example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].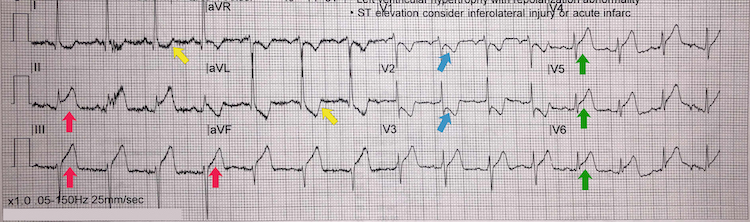 Key learning points
Key learning points
Note the deep ST-segment depression and R:S wave ratio of >1 in V1-V2 = posterior STEMI (blue arrows)
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
Reciprocal ST-segment depression in the lateral leads I and aVL (yellow arrows)
Also note the ST-segment elevation in leads V4-V6 (green arrows) – this could suggest lateral involvement of the STEMI and/or represent occlusion of a very large dominant right coronary artery with a posterior descending branch artery wrapping around the left ventricular apex
Consider performing a posterior lead ECG (leads V7-V9) for further confirmation of a posterior STEMI
Inferoposterolateral STEMI example II
[Figure caption and citation for the preceding image starts]: Inferoposterolateral STEMI example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].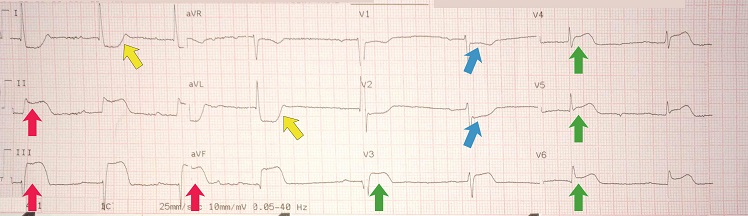 Key learning points
Key learning points
Note the deep ST-segment depression and R:S wave ratio of >1 in V1-V2 = posterior STEMI (blue arrows)
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
ST-segment elevation in leads V3-V6 = lateral STEMI (green arrows)
Reciprocal deep ST-segment depression in leads I and aVL (yellow arrows)
The patient has marked sinus bradycardia – there can be several reasons for this but with this degree of ischaemia on the ECG it suggests current or impending haemodynamic instability
Possible' inferolateral STEMI
[Figure caption and citation for the preceding image starts]: Possible’ inferolateral STEMIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].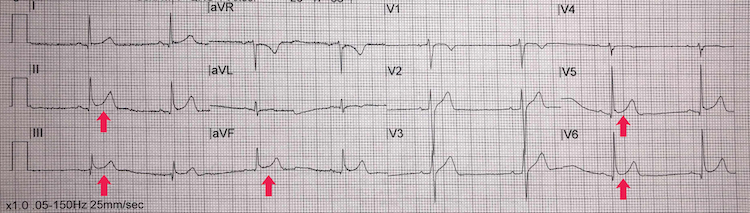 [Figure caption and citation for the preceding image starts]: Possible’ inferolateral STEMI: ST-segment shiftCreated by the BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Possible’ inferolateral STEMI: ST-segment shiftCreated by the BMJ Knowledge Centre [Citation ends].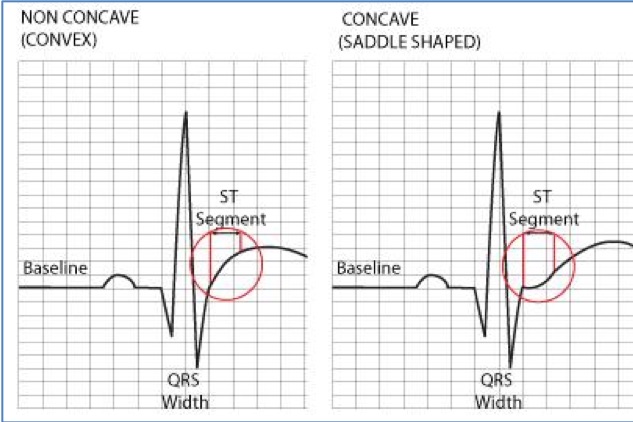
Key learning points
ST-segment elevation in the inferior leads II, III, and aVF and the lateral leads V5-V6 (red arrows)
The ST-segment shift, however, is more saddle-shaped (concave) rather than convex – see second figure above
A convex ST-segment elevation morphology is more likely to be associated with an acute myocardial infarction
There are no reciprocal ischaemic changes elsewhere on the ECG either
Take a thorough history and examination looking for the signs of myocardial ischaemia and also seek a specialist cardiology consult when managing a patient who presents with this ECG – it can still be a STEMI
Also consider acute pericarditis as a differential
Inferior STEMI example I
[Figure caption and citation for the preceding image starts]: Inferior STEMI example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].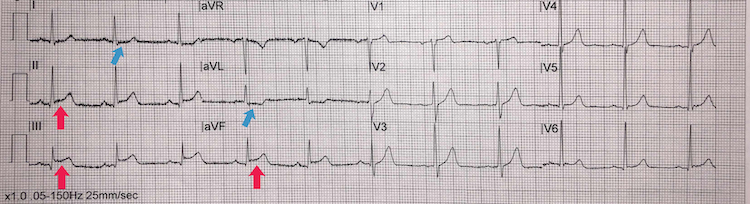 Key learning points
Key learning points
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
Subtle reciprocal ST-segment depression in the lateral leads I and aVL (blue arrows)
Inferior STEMI example II
[Figure caption and citation for the preceding image starts]: Inferior STEMI example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].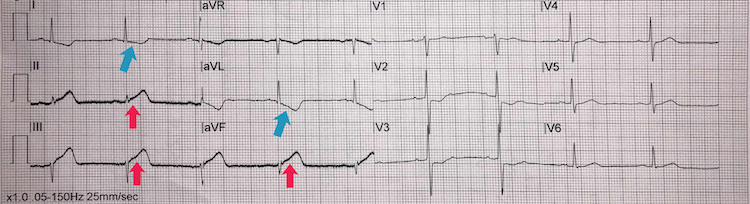 Key learning points
Key learning points
Subtle ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
Deep reciprocal ST-segment depression in the lateral leads I and aVL (blue arrows)
Take a thorough history and examination for signs and symptoms of myocardial ischaemia
Inferior STEMI example III
[Figure caption and citation for the preceding image starts]: Inferior STEMI example IIIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].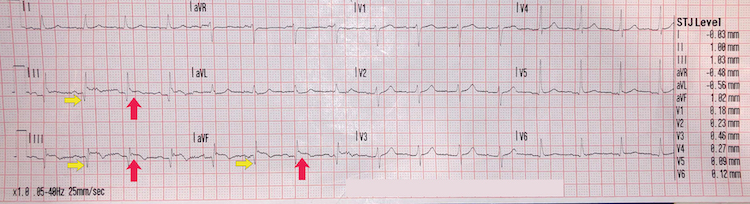 Key learning points
Key learning points
ST-segment elevation in leads II, III, and aVF = inferior STEMI (red arrows)
Pathological Q waves in leads II, III, and aVF (yellow arrows)
Note the lack of reciprocal ST-segment changes in other leads
These changes suggest a late-presentation inferior STEMI
Consult the cardiology team to discuss management options once you have performed a thorough history and examination
Paced rhythm example I
[Figure caption and citation for the preceding image starts]: Paced rhythm example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].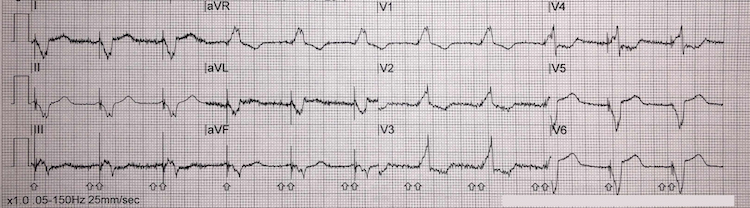 Key learning points
Key learning points
There is a right bundle branch pattern here due to cardiac pacing
Note the QRS is negative in lead I and positive in V1
There is atrial pacing here with the pacing spike preceding the P wave
There is ventricular pacing here also with the pacing spike preceding the QRS complex
Right ventricular pacing = QRS morphology similar to left bundle branch block
Left epicardial pacing = QRS morphology similar to right bundle branch block pattern
We cannot diagnose a STEMI from a paced rhythm ECG but this does not mean an acute MI can be excluded if a patient presents with the signs and symptoms of ongoing myocardial ischaemia
Paced rhythm example II
[Figure caption and citation for the preceding image starts]: Paced rhythm example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].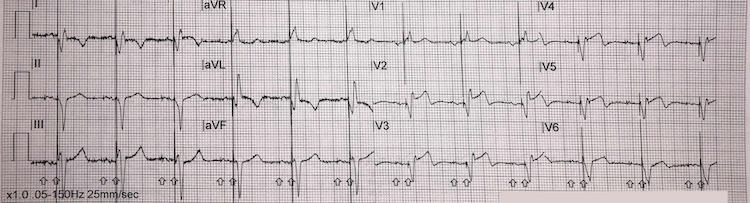 Key learning points
Key learning points
There is atrial pacing here with the pacing spike preceding the P wave
There is ventricular pacing here with the pacing spike preceding the QRS complex
We cannot diagnose a STEMI from a paced rhythm ECG but this does not mean an acute MI can be excluded if a patient presents with the signs and symptoms of ongoing myocardial ischaemia
Paced rhythm example III
[Figure caption and citation for the preceding image starts]: Paced rhythm example IIIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].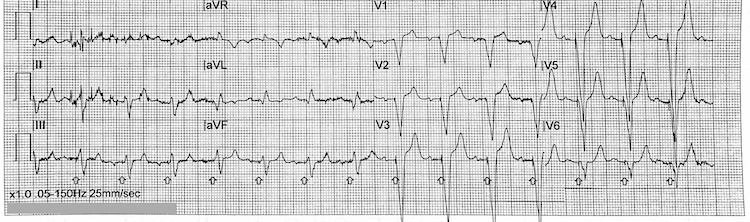 Key learning points
Key learning points
There is a bundle branch pattern here due to cardiac pacing
There is only ventricular pacing here with the pacing spike preceding the QRS complex
Right ventricular pacing = QRS morphology similar to left bundle branch block – this is the case on this ECG
We cannot diagnose a STEMI from a paced rhythm ECG but this does not mean an acute MI can be excluded if a patient presents with the signs and symptoms of ongoing myocardial ischaemia
Left ventricular hypertrophy example I
[Figure caption and citation for the preceding image starts]: Left ventricular hypertrophy example IFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends]. Key learning points
Key learning points
A STEMI cannot be diagnosed from the ECG on a background of left ventricular hypertrophy (LVH)
Numerous criteria for diagnosing LVH
Sokolov-Lyon voltage criteria: S wave depth in V1 + tallest R wave height in V5 or V6 >35 mm
Non-voltage criteria: ST-segment depression and T-wave inversion in left-sided leads I, aVL, V4-V6 (blue arrows) – this is often referred to as a left heart strain pattern
Voltage and non-voltage criteria must be present to confirm an ECG diagnosis of LVH
Left ventricular hypertrophy example II
[Figure caption and citation for the preceding image starts]: Left ventricular hypertrophy example IIFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].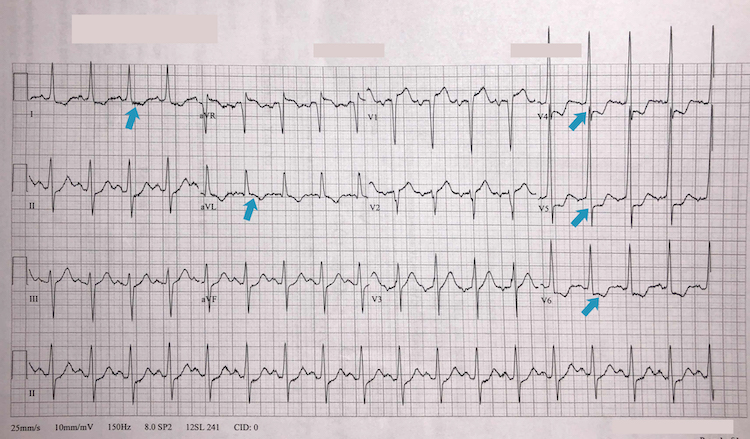 Key learning points
Key learning points
A STEMI cannot be diagnosed from the ECG on a background of left ventricular hypertrophy (LVH)
Sokolov-Lyon voltage criteria: S wave depth in V1 + tallest R wave height in V5 or V6 >35 mm
Non-voltage criteria: ST-segment depression and T-wave inversion in left-sided leads I, aVL, V4-V6 (blue arrows) – this is often referred to as a left heart strain pattern
Voltage and non-voltage criteria must be present to confirm an ECG diagnosis of LVH
Also note this type of ECG may be a normal variant in athletes and people of African-Caribbean heritage
Acute pericarditis
[Figure caption and citation for the preceding image starts]: Acute pericarditisFrom the personal collection of Dr Aung Myat (used with permission) [Citation ends].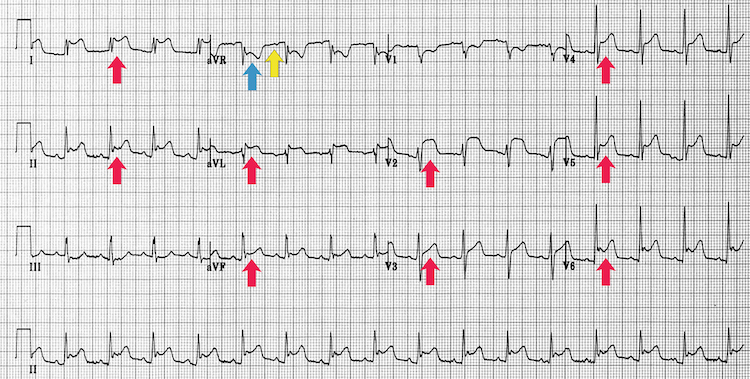 Key learning points
Key learning points
Global saddle-shaped (concave) ST-segment elevation (red arrows)
Reciprocal ST depression (blue arrow) and PR depression (yellow arrow) in leads II and aVF
Sinus tachycardia can also feature due to the increased sympathetic drive from pain and/or to maintain cardiac output if there is a large pericardial effusion associated with the inflamed pericardium
This ECG appearance does not exclude STEMI – ensure a thorough history and examination and if any doubt seek specialist help
Coronary angiography
After making a clinical diagnosis of STEMI, offer coronary angiography, with follow-on primary percutaneous coronary intervention (PCI) if indicated, as the preferred coronary reperfusion strategy, if:[75]
The patient presents with persistent typical central chest pain or other symptoms consistent with myocardial ischaemia (chest pain-equivalent symptoms) within the last 12 hours
Primary PCI can be delivered within 120 minutes of the ECG-based diagnosis.
When primary PCI is not an immediate option and cannot be provided within 120 minutes of ECG diagnosis, fibrinolysis should be initiated expeditiously as part of a pharmaco-invasive strategy, provided the patient has presented within 12 hours of symptom onset.[75]
Seek immediate specialist advice from cardiology to discuss management options for any STEMI patient who presents >12 hours after symptom onset. Coronary angiography ± primary PCI should be considered if there is evidence of continuing myocardial ischaemia, haemodynamic instability or cardiogenic shock, or life-threatening arrhythmias.[2]
If a patient with acute STEMI has had fibrinolysis, offer angiography:[75]
Immediately if an ECG 60-90 minutes after fibrinolysis shows residual ST-segment elevation (rescue PCI)
After seeking specialist cardiology advice if the patient has recurrent myocardial ischaemia after fibrinolysis
During the same hospital admission if the patient is stable after successful fibrinolysis (routine PCI strategy).
Radial arterial access is preferred to femoral access in all patients undergoing coronary angiography.[75]
Laboratory work-up
Do not delay coronary reperfusion treatment to wait for blood results.[2]
Start management as soon as a STEMI has been clinically diagnosed based on ECG findings together with signs and symptoms consistent with ongoing myocardial ischaemia.[82]
Immediately assess the patient’s eligibility for coronary reperfusion therapy (irrespective of age, ethnicity, sex, or level of consciousness).[75]
Alert the interventional cardiology team using the agreed local protocol.
Cardiac biomarkers
Request a baseline high-sensitivity cardiac troponin (cTn) along with your set of routine blood work whenever a patient presents with a possible acute MI – but do not delay coronary reperfusion if the patient has a clinical diagnosis of STEMI.[1][82]
Troponin I and T are the preferred biomarkers for definitive confirmation of an MI, with high-sensitivity assays preferred to standard ones.[1][82]
Cardiac troponins are biological markers of cardiac muscle death (cardiomyocyte necrosis) that are released into the circulation when damage to cardiac muscle has occurred.[1][89]
Creatine kinase-MB fraction is less sensitive and less specific and is now rarely used or measured.[1]
A pathological rise in troponin level followed by a later fall provides definitive confirmation of an acute MI in a patient who has clinical/ECG evidence of ongoing myocardial ischaemia.[1] However, STEMI can usually be diagnosed by ECG alone.[89]
Acute MI is definitively confirmed by a rise and/or fall in cardiac troponin (with at least one value >99th percentile of the upper reference limit) in a patient who has symptoms or signs of ischaemia.[1][82]
Troponin level deviations and normal cut-offs are assay-specific so check local protocols.
Note there is significant variability in:[1]
The time to peak value – levels usually begin to rise around 2-3 hours after onset of myocardial ischaemia but this varies according to the underlying mechanism
The time when a normal value may become greater than the 99th percentile of the upper range limit (URL)[127]
The time window to observe a fluctuating pattern of values.
Because assays are not standardised, values from one assay cannot be compared with those from a different assay.[1]
Always interpret troponin values in the context of:[82]
Current and previous ECGs
Signs and symptoms reported by the patient (in particular, the time since symptom onset)
The possibility of an alternative cause for an elevated troponin. This may be cardiac (e.g., myocarditis, aortic dissection, severe heart failure) or non-cardiac (e.g., pulmonary embolism, impaired renal function, underlying sepsis)[82]
The demonstration of a rising and/or falling pattern is important to distinguish acute myocardial injury from a chronically elevated cTn
Historical troponin levels recorded for the individual patient (e.g., measured during previous admissions)
Some patients may have a higher baseline compared with the general population.[82]
Cardiac troponin kinetics after acute myocardial injury including acute MI[1][Figure caption and citation for the preceding image starts]: Cardiac troponin kinetics after acute myocardial injury including acute MICreated by the BMJ Knowledge Centre [Citation ends].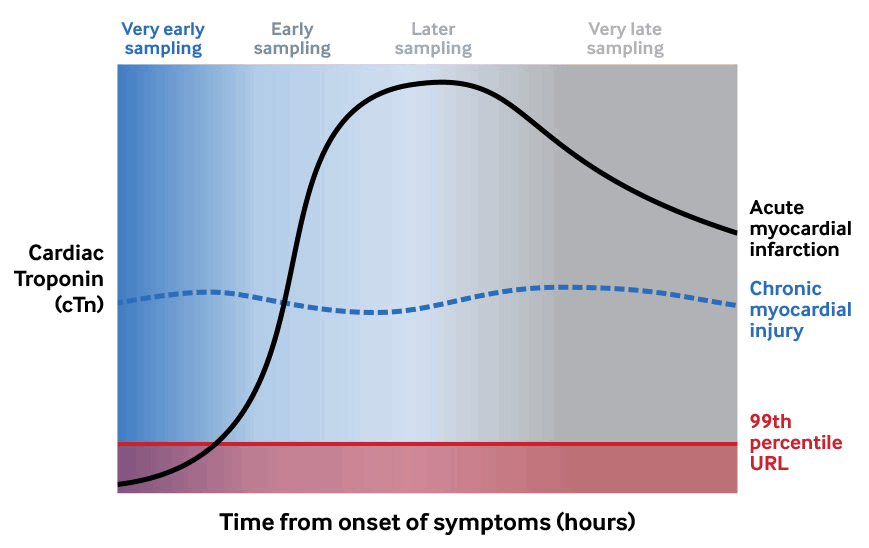
Other blood tests at presentation
Your routine blood panel should also include the following.
Full blood count
Look for anaemia, which may influence the duration of dual antiplatelet therapy prescribed.
Raised inflammatory markers may be a direct result of the acute-phase response to acute MI or may point to a concomitant infection.
Urea and electrolytes and estimated glomerular filtration rate (eGFR)
Potassium, calcium, and magnesium homeostasis is crucially important to prevent both bradyarrhythmias and tachyarrhythmias during the peri-infarct interval.
Baseline renal function at the time of hospital admission will provide a benchmark to allow for subsequent up-titration of medications and allow for the identification of contrast-induced nephropathy after primary angioplasty (if the patient is eligible for coronary reperfusion therapy).
Note patients on potentially nephrotoxic drugs on admission, especially if they are eligible for primary angioplasty.
Reduce the risk of contrast-induced nephropathy if an invasive strategy is planned in a patient with chronic kidney disease.[2]
Plasma glucose[2]
Look for uncontrolled hyperglycaemia in all patients (not just those with diabetes). Hyperglycaemia is common in the setting of acute MI, with or without a history of diabetes.
Also look for hypoglycaemia in critically ill patients.
Serum lipids
Not useful in the acute period of STEMI management but will inform assessment of the patient's risk factor profile for recurrent cardiovascular events, and aid in setting targets for lipid-lowering therapy.
C-reactive protein (CRP)
May be raised as a direct result of the acute-phase response to acute MI but may also point to a concomitant infection.
Not useful in the acute period of STEMI management but an elevated level may inform assessment of the patient’s continued risk for recurrent cardiovascular events.[128]
In a secondary analysis of the VISTA-16 trial, elevated levels of high-sensitivity CRP during the index admission and the subsequent 16 weeks after an acute coronary syndrome were associated with a higher risk of the combined end point of cardiovascular death, myocardial infarction, non-fatal stroke, unstable angina, and all-cause death.[128]
Check arterial blood gas only if there is severe dyspnoea, hypoxia, and/or clinical evidence of pulmonary oedema or cardiogenic shock, and in survivors of cardiac arrest.
Patients may require airway stabilisation and aggressive oxygen supplementation before proceeding to primary percutaneous coronary intervention.
This should not be performed routinely and must not delay coronary reperfusion therapy if there is no current or impending objective respiratory compromise.
Taking an arterial blood gas may cause trauma to the radial artery, which is typically the access point for coronary angiography.
Survivors of cardiac arrest may have:
Low PaO2
High PaCO2
PaCO2 is an independent predictor of achieving sustained return of spontaneous circulation after cardiac arrest[129]
Raised lactate
Severe metabolic acidosis.
Cardiogenic shock
Signs of acute pulmonary oedema:
Respiratory acidosis
Respiratory alkalosis (in the early stages)
Metabolic acidosis
Reduced PaO2
Reduced oxygen saturations
Acidosis is a significant predictor of mortality in acute heart failure patients.[130]
Imaging
Do not delay coronary reperfusion treatment to undertake imaging investigations.
Start treatment and assess the patient’s eligibility for coronary reperfusion therapy immediately after a clinical diagnosis of STEMI has been made.
Chest radiographs
Use a chest x-ray to exclude alternative causes and to aid indirect assessment of cardiac function.[82]
Widened mediastinum may indicate acute aortic dissection.
Pulmonary oedema suggests impaired left ventricular systolic function.
A large globular cardiac contour or significantly increased cardiothoracic ratio may suggest pericardial effusion.
Echocardiogram
Use a point-of care transthoracic echocardiogram to:[2]
Look for regional wall motion abnormalities of the left ventricle in patients with an atypical presentation or equivocal ECG[120][131]
Assess the patient’s eligibility for coronary reperfusion therapy in the event of a delayed presentation
In practice, however, the patient's clinical status and the presence of pathological Q waves in the ECG are usually enough to assess their eligibility for coronary reperfusion therapy if presentation is delayed
Look for mechanical complications of acute MI (see Acute MI complications – mechanical below):[131]
Left ventricular function
Right ventricular function
Ventricular septal rupture
Left ventricular free wall rupture
Acute mitral regurgitation
Pericardial effusion
Cardiac tamponade.
An echocardiogram can also be used to:
Exclude STEMI in patients who present with global saddle-shaped ST-segment elevation (as seen with acute pericarditis)[131]
Confirm the diagnosis of takotsubo cardiomyopathy (usually after normal coronary arteries are found on a STEMI angiogram)[1][131]
Suggest alternative aetiologies associated with chest pain (e.g., acute aortic disease, pulmonary embolism).[2][131]
A pre-discharge echocardiogram is indicated for all patients post-acute MI to assess left ventricular function after coronary reperfusion therapy and to guide prognostication.[71][75]
Emerging investigations
Cardiac myosin-binding protein C (cMyC)
CMyC may perform better than cardiac troponin T or I in patients who present early after symptom onset.[132]
CMyC is a cardiac-restricted protein that is released more rapidly than cardiac troponin after acute MI.
CMyC is also more abundant than cardiac troponins.
It may become the gold standard test for the early diagnosis of acute MI, though is not currently used in clinical practice.
Risk stratification scores for STEMI are of limited use in the emergency department setting.
This is largely because the emphasis should be on rapid triage based largely on ECG diagnosis, and assessment of eligibility for immediate coronary reperfusion therapy.
Nonetheless, risk scores can:
Provide evidence-based prognostic information
Be used to support decisions on the optimum reperfusion strategy.
The European Society of Cardiology recommends the GRACE risk score for acute coronary syndrome.[2][133][134] [ GRACE Score for Acute Coronary Syndrome Prognosis Opens in new window ] Note that the GRACE score is of less value in STEMI than in non-STEMI, where it used for triage and to guide timing of invasive intervention.
Left ventricular dysfunction[2][71]
Severity depends on the duration of ischaemia, premorbid functional state, and presence of concomitant mechanical complications of acute MI.
Can be transient (myocardial stunning) or persistent.
May be clinically silent or lead to symptoms and signs of heart failure.
Left ventricular aneurysm[71]
Affects <5% of those with large transmural MIs (especially anterior STEMI)
May present with signs of heart failure, ventricular arrhythmias, or clinical sequelae of thromboembolism.
ECG changes can support clinical suspicion.
Will require imaging to confirm the diagnosis.
Left ventricular thrombus[2][71]
Relatively frequent complication of large anterior STEMI.[137]
No specific clinical signs.
May present with signs of heart failure or sequelae of systemic thromboembolism.
(Contrast) echocardiography required to confirm diagnosis.
Right ventricular infarction[71]
May present with the triad of hypotension, elevated jugular venous pressure (JVP), and clear lung fields.[83]
Can complicate up to one third of inferior STEMIs.
Check for ST elevation in aVR, V1, and right precordial leads.
Confirm the diagnosis with echocardiography.
Ventricular septal rupture[2][71]
Presents with acute heart failure or cardiogenic shock.
Look for a loud systolic murmur.
Rupture after anterior STEMI = apical ventricular septal defect (VSD).
Rupture after inferior STEMI = basal VSD (worse prognosis).
Resulting left-to-right shunt may give rise to signs of acute right heart failure.
The diagnosis is confirmed by echocardiography and Doppler (to quantify the degree of shunting).
Left ventricular free wall rupture[2][71]
Presents with sudden-onset chest pain and/or haemodynamic collapse.
Rupture leads to haemopericardium and ultimately tamponade.
Followed by electromechanical dissociation and typically death.
Mortality rates range from 20% to 95%.[138]
More common after first MI, anterior STEMI, lack of reperfusion, or late fibrinolysis, and in elderly patients and women.
The diagnosis is confirmed by echocardiography.
Acute mitral regurgitation (MR)[2][71]
Secondary to:
Papillary muscle rupture or
Functional/ischaemic’ MR
Post-infarction left ventricular remodelling characterised by: papillary muscle displacement, leaflet tethering, and annular dilatation
Look for signs of severe dyspnoea, acute pulmonary oedema, and/or cardiogenic shock.
Classical pansystolic murmur of mitral regurgitation may not always be audible due to the severity of the regurgitation.
Inferior STEMI can cause rupture of the posteromedial papillary muscle.
Anterolateral STEMI can cause rupture of the anterolateral papillary muscle.
The diagnosis is confirmed by echocardiography.
Diagnostic criteria:[2]
Pleuritic chest pain (relieved by sitting or leaning forward)
Pericardial friction rub
ECG changes: global ST-segment elevation or PR interval depression
Pericardial effusion (new or worsening).
Early post-MI pericarditis: usually transient.
Late post-MI pericarditis: also known as Dressler syndrome; more common after late-presentation STEMI.
Both are related to late or failed reperfusion and larger infarct size.
Secondary to pericarditis.
Can be a complication of primary percutaneous coronary intervention.
In the absence of inflammatory signs: rule out subacute left ventricular free wall rupture.
Look for signs of cardiac tamponade:
Beck’s triad = hypotension with narrow pulse pressure + raised JVP + muffled heart sounds
Pulsus paradoxus = exaggerated fall in systolic pressure >10 mmHg during inspiration
Electrical alternans (and tachycardia) on ECG
Pleuritic chest pain
Tachypnoea
Weakness, anxiety, restlessness.
Use echocardiography first-line if there is a high clinical suspicion of pericardial effusion and/or tamponade.
Bradycardia and atrioventricular block[71]
Sinus bradycardia is common post-STEMI and is usually self-limiting.
Mobitz type I second-degree atrioventricular (AV) block is associated with inferior STEMI.
Rarely causes haemodynamic compromise.
Mobitz type II second-degree AV block and complete heart block:
May require pacing.
Practical tip
AV block secondary to inferior STEMI quite often resolves spontaneously or after coronary reperfusion.
AV block secondary to anterior STEMI is associated with a high mortality rate and indicates an extensive area of myocardial damage. It will need consideration of permanent pacemaker implantation.
Left bundle branch block or hemiblock suggests an extensive anterior MI.
Supraventricular tachyarrhythmias[2][71]
Atrial fibrillation most common.
Determine the patient’s haemodynamic status and treat accordingly.
Ventricular tachyarrhythmias[2][71]
Secondary to ischaemia: unstable, polymorphic, relatively fast ventricular tachycardia degenerating into ventricular fibrillation.
Secondary to reperfusion (within 48 hours): ventricular extrasystoles, non-sustained ventricular tachycardia.
Usually benign if self-limiting.
Be aware of incessant ventricular tachycardia/electrical storm despite appropriate revascularisation.
It is crucial to identify and treat sustained ventricular arrhythmias in the peri-infarction period to prevent:
Further reduction in cardiac output
Worsening myocardial ischaemia
Degeneration into ventricular fibrillation
Use of this content is subject to our disclaimer