Aetiology
The aetiology of polycystic ovary syndrome (PCOS) is unknown. It is a syndrome wherein multiple systems are affected and the site of the primary defect is unclear. Various lines of evidence have supported primary defects in the hypothalamic-pituitary axis, postulating increased amplitude and frequency of pulses of luteinising hormone (LH), or defects involving the ovaries through an intrinsic problem leading to androgen over-production. Some theories postulate defects in insulin sensitivity with insulin resistance leading to compensatory hyper-insulinaemia.
PCOS appears to be inherited as a common complex disorder. Multiple genes, each with mild or moderate effects on overall disease risk, are likely to be involved.[17] One twin study calculated the heritability of PCOS at 70%, suggesting that most PCOS risk depends on genetic factors.[18] The actual PCOS susceptibility genes have yet to be definitively identified. A large genome-wide association study conducted in Chinese individuals robustly identified genetic variants in or near 3 genes (DENND1A, THADA, LHCGR) as risk factors for PCOS.[19] Variation in DENND1A was subsequently associated with PCOS in several European-origin cohorts.[20][21][22][23] A subsequent genome-wide association study in Chinese individuals increased the number of susceptibility loci to 11.[24] Three genome-wide association studies in predominantly European-origin cohorts increased the total number of PCOS susceptibility variants to 25.[25][26][27] Most loci discovered in one ethnic group appear to affect PCOS risk in other ethnic groups, suggesting an ancient origin of PCOS.[28] Susceptibility loci include genes for the LH receptor, the follicle-stimulating hormone receptor, and the follicle-stimulating hormone beta sub-unit, suggesting a key role for disordered gonadotrophin function in PCOS. Epigenetic changes (DNA alterations independent of the primary nucleotide sequence; e.g., DNA methylation) may also play a role in PCOS susceptibility, as evidenced by differential X-chromosome inactivation in PCOS.[29][30]
Pathophysiology
The pathophysiology of PCOS is not well understood, mainly due to lack of knowledge of the location of the primary defect. There are several candidates: ovary, adrenal, hypothalamus, pituitary, or insulin-sensitive tissues. It is possible that there are subsets of women with PCOS wherein each of these proposed mechanisms serves as the primary defect.[Figure caption and citation for the preceding image starts]: Simplified diagram of the main pathogenic factors in PCOS. ACTH, adrenocorticotrophic hormone; FSH, follicle-stimulating hormone; GnRH, gonadotrophin-releasing hormone; LH, luteinising hormoneFrom the collection of Dr M.O. Goodarzi; used with permission [Citation ends].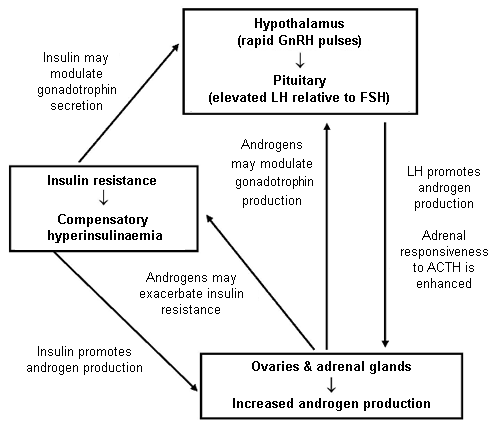
Investigations have elucidated some of the interactions between these systems. Insulin resistance leads to compensatory insulin hypersecretion by the pancreas in order to maintain normoglycaemia. The resulting hyperinsulinaemia promotes ovarian androgen output and may also promote adrenal androgen output. High insulin levels also suppress hepatic production of sex hormone-binding globulin, which exacerbates hyper-androgenaemia by increasing the proportion of free circulating androgens.[12] Another factor that promotes ovarian androgen output is the fact that women with PCOS are exposed long term to high levels of LH. This LH excess seems to be a result of an increased frequency of gonadotrophin-releasing hormone pulses from the hypothalamus.[12][31] The abnormal hormonal milieu may also contribute to incomplete follicular development that results in polycystic ovarian morphology.[12]
Classification
Proposed classification[5][6]
There is no formal classification system for PCOS. The following has been proposed:
Mild PCOS (accounts for 16% of affected women): characterised by irregular periods, polycystic ovaries on ultrasound, mildly elevated androgen concentrations, normal insulin concentrations, unknown long-term risks
Ovulatory PCOS (accounts for 16% of affected women): characterised by normal periods, polycystic ovaries on ultrasound, elevated androgen concentrations, increased insulin concentrations, unknown long-term risks
Hyper-androgenism and chronic anovulation (accounts for 7% of affected women): characterised by irregular periods, normal ovaries on ultrasound, elevated androgen concentrations, increased insulin concentrations, potential long-term risks
Severe PCOS (accounts for 61% of affected women): characterised by irregular periods, polycystic ovaries on ultrasound, elevated androgen concentrations, increased insulin concentrations, potential long-term risks.
Use of this content is subject to our disclaimer