Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
acute gout
non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or colchicine are recommended first-line treatments for patients experiencing a gout flare.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [82]Billy CA, Lim RT, Ruospo M, et al. Corticosteroid or nonsteroidal antiinflammatory drugs for the treatment of acute gout: a systematic review of randomized controlled trials. J Rheumatol. 2018 Jan;45(1):128-36. https://www.jrheum.org/content/45/1/128.long http://www.ncbi.nlm.nih.gov/pubmed/28765243?tool=bestpractice.com The choice between treatments should be guided by patient preference and potential risks and contraindications (e.g., renal impairment in the case of NSAIDs).[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
NSAIDs halt the inflammatory cascade if they are started early. Also used to suppress gouty attacks when maintenance therapy with urate-lowering drugs is started.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
It is common to use indometacin, although there is no evidence to suggest that it is more effective than other NSAIDs.[84]Qaseem A, Harris RP, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58-68. https://www.acpjournals.org/doi/full/10.7326/M16-0570 http://www.ncbi.nlm.nih.gov/pubmed/27802508?tool=bestpractice.com
One Cochrane review reported, with moderate certainty, that NSAIDs and selective cyclo-oxygenase-2 (COX-2) inhibitors have similar efficacy for pain, swelling, treatment success, and quality of life in patients with gout.[85]van Durme CM, Wechalekar MD, Landewé RB, et al. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2021 Dec 9;12:CD010120.
https://www.doi.org/10.1002/14651858.CD010120.pub3
http://www.ncbi.nlm.nih.gov/pubmed/34882311?tool=bestpractice.com
[  ]
For people with acute gout, how do non‐steroidal anti‐inflammatory drugs (NSAIDs) compare with cyclo‐oxygenase (COX)‐2 inhibitors (COXIBs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3940/fullShow me the answer The review found no difference between groups for function, but this result was based on low-certainty evidence. Higher withdrawal rates due to adverse events (mainly gastrointestinal) were seen with non-selective NSAIDs.[85]van Durme CM, Wechalekar MD, Landewé RB, et al. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2021 Dec 9;12:CD010120.
https://www.doi.org/10.1002/14651858.CD010120.pub3
http://www.ncbi.nlm.nih.gov/pubmed/34882311?tool=bestpractice.com
[
]
For people with acute gout, how do non‐steroidal anti‐inflammatory drugs (NSAIDs) compare with cyclo‐oxygenase (COX)‐2 inhibitors (COXIBs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3940/fullShow me the answer The review found no difference between groups for function, but this result was based on low-certainty evidence. Higher withdrawal rates due to adverse events (mainly gastrointestinal) were seen with non-selective NSAIDs.[85]van Durme CM, Wechalekar MD, Landewé RB, et al. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2021 Dec 9;12:CD010120.
https://www.doi.org/10.1002/14651858.CD010120.pub3
http://www.ncbi.nlm.nih.gov/pubmed/34882311?tool=bestpractice.com
[  ]
For people with acute gout, how do non‐steroidal anti‐inflammatory drugs (NSAIDs) compare with cyclo‐oxygenase (COX)‐2 inhibitors (COXIBs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3940/fullShow me the answer An additional systematic review concluded that although COX-2 inhibitors are equally as effective as non-selective NSAIDs for pain relief in patients with gout, COX-2 inhibitors may be more beneficial overall.[86]Li M, Yu C, Zeng X. Comparative efficacy of traditional non-selective NSAIDs and selective cyclo-oxygenase-2 inhibitors in patients with acute gout: a systematic review and meta-analysis. BMJ Open. 2020 Sep 10;10(9):e036748.
https://www.doi.org/10.1136/bmjopen-2019-036748
http://www.ncbi.nlm.nih.gov/pubmed/32912981?tool=bestpractice.com
]
For people with acute gout, how do non‐steroidal anti‐inflammatory drugs (NSAIDs) compare with cyclo‐oxygenase (COX)‐2 inhibitors (COXIBs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3940/fullShow me the answer An additional systematic review concluded that although COX-2 inhibitors are equally as effective as non-selective NSAIDs for pain relief in patients with gout, COX-2 inhibitors may be more beneficial overall.[86]Li M, Yu C, Zeng X. Comparative efficacy of traditional non-selective NSAIDs and selective cyclo-oxygenase-2 inhibitors in patients with acute gout: a systematic review and meta-analysis. BMJ Open. 2020 Sep 10;10(9):e036748.
https://www.doi.org/10.1136/bmjopen-2019-036748
http://www.ncbi.nlm.nih.gov/pubmed/32912981?tool=bestpractice.com
Choice of NSAID should be guided by patient preference.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
In patients at high risk of gastrointestinal complications, a proton-pump inhibitor or misoprostol should be co-prescribed.
Proton-pump inhibitors should be considered for all patients who are taking an NSAID.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
COX-2 inhibitors may be safer than traditional NSAIDs in patients with a history of gastrointestinal bleeding or comorbidities.
Treatment course is generally 7 to 14 days. However, NSAIDs should be given for the shortest period possible, at the lowest effective dose.
Primary options
naproxen: 500 mg orally twice daily
OR
ibuprofen: 400-800 mg orally three to four times daily
OR
diclofenac potassium: 50 mg orally (immediate-release) three times daily
OR
meloxicam: 7.5 to 15 mg orally once daily
OR
indometacin: 25-50 mg orally three times daily
OR
celecoxib: 100-200 mg orally twice daily
corticosteroid
Corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), or colchicine are recommended first-line treatments for patients experiencing a gout flare.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [82]Billy CA, Lim RT, Ruospo M, et al. Corticosteroid or nonsteroidal antiinflammatory drugs for the treatment of acute gout: a systematic review of randomized controlled trials. J Rheumatol. 2018 Jan;45(1):128-36. https://www.jrheum.org/content/45/1/128.long http://www.ncbi.nlm.nih.gov/pubmed/28765243?tool=bestpractice.com
The choice between treatments should be guided by patient preference and potential risks and contraindications (e.g., renal impairment in the case of NSAIDs).[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Corticosteroids can be given either as an intra-articular injection for monoarticular acute gout or parenterally for oligoarticular or polyarticular acute gout.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com Parenteral dose depends on the severity of the presentation; switch to oral dosing after 1-2 days and then taper oral dose as usual.
In the UK, off-label use of intra-articular or intramuscular injections of corticosteroids are only considered if NSAIDs and colchicine are contraindicated.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Corticosteroids are probably more effective than colchicine for acute gout, although there are no head-to-head trials.[87]Curiel RV, Guzman NJ. Challenges associated with the management of gouty arthritis in patients with chronic kidney disease: a systematic review. Semin Arthritis Rheum. 2012;42:166-178. http://www.ncbi.nlm.nih.gov/pubmed/22560299?tool=bestpractice.com
Corticosteroids are associated with potentially serious adverse effects.
One Cochrane review reported moderate-certainty evidence that corticosteroids had similar efficacy for pain, inflammation, function, and treatment success when compared with NSAIDs for patients with gout.[85]van Durme CM, Wechalekar MD, Landewé RB, et al. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2021 Dec 9;12:CD010120. https://www.doi.org/10.1002/14651858.CD010120.pub3 http://www.ncbi.nlm.nih.gov/pubmed/34882311?tool=bestpractice.com
Should be avoided if septic arthritis has not been excluded.
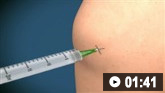
How to aspirate synovial fluid from the knee and administer intra-articular medication using a medial approach.
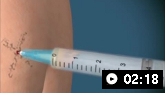
How to aspirate synovial fluid from the shoulder and administer intra-articular medication. Video demonstrates a posterior approach to the glenohumeral joint and a lateral approach to the subacromial space.
Primary options
prednisolone: 1 mg/kg orally given as a single dose
More prednisoloneThis regimen may be adequate if started within 24 hours of attack.
OR
prednisolone: 20-40 mg orally once daily initially, decrease by 5-10 mg/day decrements every 3 days until discontinuation; or 30 mg orally once daily for 5 days
OR
methylprednisolone acetate: small joint: 4-10 mg intra-articularly as a single dose; medium joint: 10-40 mg intra-articularly as a single dose; large joint: 20-80 mg intra-articularly as a single dose; consult specialist for guidance on parenteral dose
OR
triamcinolone acetonide: small joint: 2.5 to 10 mg intra-articularly as a single dose; larger joint: 5-40 mg intra-articularly as a single dose
colchicine
Colchicine, non-steroidal anti-inflammatory drugs (NSAIDs), or corticosteroids are recommended first-line treatments for patients experiencing a gout flare.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [82]Billy CA, Lim RT, Ruospo M, et al. Corticosteroid or nonsteroidal antiinflammatory drugs for the treatment of acute gout: a systematic review of randomized controlled trials. J Rheumatol. 2018 Jan;45(1):128-36. https://www.jrheum.org/content/45/1/128.long http://www.ncbi.nlm.nih.gov/pubmed/28765243?tool=bestpractice.com
The choice between treatments should be guided by patient preference and potential risks and contraindications (e.g., renal impairment in the case of NSAIDs).[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Minimal effective dose of colchicine should be used because of the narrow benefit to risk index.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com The conclusion of one Cochrane review, based on low-quality evidence, suggests that low-dose colchicine may be effective for the treatment of gout compared with placebo, and may have similar efficacy compared with NSAIDs.[88]McKenzie BJ, Wechalekar MD, Johnston RV, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2021 Aug 26;8:CD006190. https://www.doi.org/10.1002/14651858.CD006190.pub3 http://www.ncbi.nlm.nih.gov/pubmed/34438469?tool=bestpractice.com
Common adverse effects are diarrhoea, nausea, and vomiting.[89]Stewart S, Yang KCK, Atkins K, et al. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020 Feb 13;22(1):28. https://www.doi.org/10.1186/s13075-020-2120-7 http://www.ncbi.nlm.nih.gov/pubmed/32054504?tool=bestpractice.com In rare cases, colchicine treatment has been associated with an increased risk for fetal chromosomal aberrations.[90]Singer A, Grinshpun-Cohen J, Sagi-Dain L. Colchicine treatment increases the risk for fetal chromosomal aberrations - an observational study and systematic literature review. Rheumatology (Oxford). 2021 May 14;60(5):2342-7. https://www.doi.org/10.1093/rheumatology/keaa602 http://www.ncbi.nlm.nih.gov/pubmed/33179053?tool=bestpractice.com
Give until relief, nausea/vomiting, diarrhoea, or maximum dose reached; diarrhoea will probably precede pain relief; wait 3 days between courses.
Primary options
colchicine: 1.2 mg orally initially, followed by 0.6 mg orally after 1 hour, maximum 1.8 mg total dose
interleukin (IL) -1 inhibitor
Anakinra and canakinumab are second-line therapies. They are conditionally recommended over no therapy (beyond supportive/analgesic treatment) for patients experiencing a gout flare who are refractory to, are intolerant of, or have contraindications to other anti-inflammatory therapies.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Anakinra, a recombinant IL-1 receptor antagonist, has been found to be non-inferior to usual treatment (patient choice of either colchicine, naproxen, or prednisolone) for the management of acute gout flare.[91]Janssen CA, Oude Voshaar MAH, Vonkeman HE, et al. Anakinra for the treatment of acute gout flares: a randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial. Rheumatology (Oxford). 2019 Jan 2 [Epub ahead of print]. https://www.jrheum.org/content/45/1/128.long http://www.ncbi.nlm.nih.gov/pubmed/30602035?tool=bestpractice.com In patients for whom a non-steroidal anti-inflammatory drug (NSAID) and colchicine was ineffective or contraindicated, treatment with either anakinra or the corticosteroid triamcinolone reduced patient-assessed gout flare pain to a similar extent within 72 hours.[92]Saag K, So A, Khanna P, et al. THU0409 a randomized, phase 2 study evaluating the efficacy and safety of anakinra in difficult-to-treat acute gouty arthritis: the anaGO study. 2020;79 (Suppl 1):442. https://ard.bmj.com/content/79/Suppl_1/442.1 Anakinra is off-label for the treatment of gout in the US and Europe.
Canakinumab is an IL-1-beta inhibitor monoclonal antibody. Pooled results from two randomised, double-blind trials indicate that patients who received a single dose of canakinumab during an acute flare experienced rapid and effective pain relief compared with patients receiving triamcinolone (mean 72-hour visual analogue scale pain score of 25.0 mm vs. 35.7 mm, respectively; P <0.0001), and a 56% reduction in risk of new flares over a 24-week period (hazard ratio 0.44; P ≤0.0001).[93]Schlesinger N, Alten RE, Bardin T, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71:1839-1848. http://www.ncbi.nlm.nih.gov/pubmed/22586173?tool=bestpractice.com
The US Food and Drug Administration panel rejected the approval of canakinumab for the treatment of gout due to the potential risk of infection, hypertriglyceridaemia, and elevated uric acid levels.[94]US Food and Drug Administration. FDA briefing document supplemental BLA 125319: Ilaris (canakinumab) for the following proposed indication: "ILARIS is indicated for the treatment of gouty arthritis attacks. ILARIS has also been shown to extend the time to the next attack and reduce the frequency of subsequent attacks."June 2011 [internet publication].
Canakinumab is approved by the European Medicines Agency for the symptomatic treatment of adult patients with frequent gouty arthritis attacks (at least three attacks in the previous 12 months) in whom NSAIDs and colchicine are contraindicated, are not tolerated, or do not provide an adequate response, and in whom repeated courses of corticosteroids are not appropriate.
Primary options
anakinra: consult specialist for guidance on dose
OR
canakinumab: consult specialist for guidance on dose
recurrent gout: 2-3 weeks post acute episode
allopurinol
Initiation of treatment with urate-lowering drugs (e.g., xanthine oxidase inhibitors such as allopurinol and febuxostat; uricosuric agents such as probenecid or pegloticase) is recommended for patients with gout with ≥1 subcutaneous tophi, evidence of radiographic damage (any modality) attributable to gout, or frequent gout flares (defined as ≥2 annually).[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Urate-lowering drugs may also be used to treat: patients who have experienced at least one previous gout flare but have infrequent flares (<2/year); patients experiencing their first flare who have comorbid moderate-to-severe chronic kidney disease (stage ≥3), serum urate concentration >540 micromol/L (>9 mg/dL), or urolithiasis; patients who receive diuretic therapy; and patients who have chronic gouty arthritis.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Patients experiencing their first gout flare, and those with asymptomatic hyperuricaemia, should not be treated with urate-lowering drugs.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
In the US, treatment can be initiated during a gout flare.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [Evidence C]5948329f-76c9-4ea0-9f81-8f729694a5f2guidelineCWhat are the effects of urate-lowering therapy (ULT)ᵃ during a gout flare compared with ULT after a gout flare has resolved in people diagnosed with gout?[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com There is evidence to suggest that starting urate-lowering therapy during a flare does not significantly extend flare duration or severity.[99]Hill EM, Sky K, Sit M, et al. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. J Clin Rheumatol. 2015 Apr;21(3):120-5. http://www.ncbi.nlm.nih.gov/pubmed/25807090?tool=bestpractice.com [100]Taylor TH, Mecchella JN, Larson RJ, et al. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med. 2012 Nov;125(11):1126-34. http://www.ncbi.nlm.nih.gov/pubmed/23098865?tool=bestpractice.com Furthermore, symptoms related to the current flare may motivate patients to take urate-lowering therapy (ULT). The decision to initiate ULT should be based on patient preference.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com However, in the UK, it is recommended that ULT is started at least 2 to 4 weeks after a gout flare has settled, unless the patient is experiencing frequent flares, in which case ULT can be started during a flare.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Urate-lowering drugs are typically prescribed to target serum uric acid levels <360 micromol/L (<6 mg/dL), to prevent supersaturation and crystal formation.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com In the UK, a lower target serum urate level below 300 micromol/L (5 mg/dL) should be considered for patients who have tophi or chronic gouty arthritis, or who continue to have frequent flares despite having a serum urate level below 360 micromol/L (6 mg/dL).[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
One double blind randomised controlled trial demonstrated that more intensive serum urate lowering does not improve bone erosion scores in patients with erosive gout at 2 years.[101]Dalbeth N, Doyle AJ, Billington K, et al. Intensive serum urate lowering with oral urate-lowering therapy for erosive gout: a randomized double-blind controlled trial. Arthritis Rheumatol. 2022 Jun;74(6):1059-69. https://www.doi.org/10.1002/art.42055 http://www.ncbi.nlm.nih.gov/pubmed/34927391?tool=bestpractice.com
Allopurinol is the preferred first-line urate-lowering agent for all patients with gout, including those with moderate-to-severe chronic kidney disease (stage ≥3).[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [104]Wei J, Choi HK, Neogi T, et al. Allopurinol initiation and all-cause mortality among patients with gout and concurrent chronic kidney disease : a population-based cohort study. Ann Intern Med. 2022 Apr;175(4):461-70. https://www.doi.org/10.7326/M21-2347 http://www.ncbi.nlm.nih.gov/pubmed/35073156?tool=bestpractice.com
In the UK, allopurinol is recommended as joint first line treatment with febuxostat, however, for patients with major cardiovascular disease (e.g., previous myocardial infarction, stroke, or unstable angina) allopurinol should be offered as first line treatment.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
It can be started at a low dose (≤100 mg/day and lower in patients with chronic kidney disease [stage ≥3]) during a gout flare, or once the flare has abated depending on patient preference.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
The dose should be increased over several weeks to months until the uric acid level is <360 micromol/L (<6 mg/dL).[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Evidence from one randomised controlled trial indicates that, in addition to reducing monosodium urate crystal burden, long-term allopurinol can slow the progression of bone erosion using a treat‐to‐serum urate target (<360 micromol/L [<6 mg/dL]) strategy.[105]Dalbeth N, Billington K, Doyle A, et al. Effects of allopurinol dose escalation on bone erosion and urate volume in gout: a dual-energy computed tomography imaging study within a randomized, controlled trial. Arthritis Rheumatol. 2019 Oct;71(10):1739-46. http://www.ncbi.nlm.nih.gov/pubmed/31081595?tool=bestpractice.com
In the UK, if the target serum urate level is not reached or first line treatment with allopurinol is not tolerated, switching to a second line treatment with febuxostat should be considered, taking into account any comorbidities or preferences.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Allopurinol should be initiated at lower doses in patients with renal insufficiency because of the risk of allopurinol hypersensitivity.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com Gradual allopurinol dose escalation, in conjunction with kidney and liver function monitoring, may be considered in patients with kidney impairment.[106]Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536. http://www.ncbi.nlm.nih.gov/pubmed/22488501?tool=bestpractice.com
Prospective cohort studies suggest that allopurinol therapy is associated with a reduced incidence of renal disease.[107]Vargas-Santos AB, Peloquin CE, Zhang Y, et al. Association of chronic kidney disease with allopurinol use in gout treatment. JAMA Intern Med. 2018 Nov 1;178(11):1526-33. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2705694 http://www.ncbi.nlm.nih.gov/pubmed/30304329?tool=bestpractice.com [108]Krishnan E, Akhras KS, Sharma H, et al. Serum urate and incidence of kidney disease among veterans with gout. J Rheumatol. 2013;40:1166-1172. https://www.jrheum.org/content/40/7/1166.long http://www.ncbi.nlm.nih.gov/pubmed/23678154?tool=bestpractice.com
In populations where HLA-B*5801-positive people are at high risk for severe allopurinol hypersensitivity reaction (e.g., Koreans with renal insufficiency, Han Chinese descent, and Thai descent), HLA-B*5801 screening may be considered.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com One large retrospective study conducted in Taiwan estimated the annual incidence of hypersensitivity reaction in new users of allopurinol at 4.68 per 1000, with mortality of 0.39 per 1000.[109]Yang CY, Chen CH, Deng ST, et al. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan. JAMA Intern Med. 2015;175:1550-1557. http://www.ncbi.nlm.nih.gov/pubmed/26193384?tool=bestpractice.com The risk of hypersensitivity was statistically significant among patients with renal or cardiovascular disease who were prescribed allopurinol for asymptomatic hyperuricemia.[109]Yang CY, Chen CH, Deng ST, et al. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan. JAMA Intern Med. 2015;175:1550-1557. http://www.ncbi.nlm.nih.gov/pubmed/26193384?tool=bestpractice.com A subsequent Taiwanese cohort study found that allopurinol is associated with a 5.5-fold increased risk of cutaneous adverse reactions compared with febuxostat.[110]Lin CW, Huang WI, Chao PH, et al. Risk of cutaneous adverse reactions associated with allopurinol or febuxostat in real-world patients: a nationwide study. Int J Clin Pract. 2019 May;73(5):e13316. http://www.ncbi.nlm.nih.gov/pubmed/30681751?tool=bestpractice.com
Primary options
allopurinol: 100 mg orally once daily initially, increase by 100 mg/day increments every week according to serum urate level, maximum 800 mg/day; a lower starting dose may be required in patients with renal impairment
suppressive therapy
Treatment recommended for ALL patients in selected patient group
Suppressive (prophylactic) therapy such as non-steroidal anti-inflammatory drugs (NSAIDs), low-dose colchicine, or low dose oral corticosteroid should be considered during initiation and tapering of a urate-lowering agent.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [84]Qaseem A, Harris RP, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58-68. https://www.acpjournals.org/doi/full/10.7326/M16-0570 http://www.ncbi.nlm.nih.gov/pubmed/27802508?tool=bestpractice.com
The therapy should be continued for at least 3 to 6 months after reaching the target level of serum uric acid.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Prednisolone may be considered if both NSAIDs and colchicine are contraindicated, at the lowest dose to prevent gout flares.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Primary options
naproxen: 250 mg orally twice daily
OR
ibuprofen: 400 mg orally three times daily
OR
diclofenac potassium: 50 mg orally (immediate-release) two to three times daily
OR
meloxicam: 7.5 to 15 mg orally once daily
OR
indometacin: 25 mg orally three times daily
OR
celecoxib: 100-200 mg orally once daily
OR
colchicine: 0.6 mg orally once daily
Secondary options
prednisolone: 7.5 to 10 mg orally once daily initially, adjust to lowest dose that prevents gout flares
febuxostat
Febuxostat is a non-purine selective xanthine oxidase inhibitor that reduces the production of uric acid.
[  ]
Does febuxostat improve outcomes in people with chronic gout?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.186/fullShow me the answer
]
Does febuxostat improve outcomes in people with chronic gout?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.186/fullShow me the answer
The US Food and Drug Administration recommends that febuxostat should only be prescribed for patients who have failed treatment with, or cannot tolerate, allopurinol and who have been counselled regarding the cardiovascular risk.[111]US Food and Drug Administration. FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat). Feb 2019 [internet publication]. https://www.fda.gov/Drugs/DrugSafety/ucm631182.htm
American College of Rheumatology guidelines recommend that patients with gout who are taking febuxostat, and have a history of cardiovascular disease or a new cardiovascular event, should switch to another urate-lowering therapy, if available.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
The Medicines and Healthcare Products Regulatory Agency in the UK recommends that patients with pre-existing major cardiovascular disease (e.g., myocardial infarction, stroke, or unstable angina) should avoid treatment with febuxostat unless no other therapy options are available.[112]Medicines and Healthcare products Regulatory Agency. Febuxostat (Adenuric): increased risk of cardiovascular death and all-cause mortality in clinical trial in patients with a history of major cardiovascular disease. Jul 2019 [internet publication]. https://www.gov.uk/drug-safety-update/febuxostat-adenuric-increased-risk-of-cardiovascular-death-and-all-cause-mortality-in-clinical-trial-in-patients-with-a-history-of-major-cardiovascular-disease
Febuxostat is recommended as joint first line treatment with allopurinol in the UK, once the patients comorbidities and preferences are noted.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 If the target serum urate level is not reached or first line treatment with febuxostat is not tolerated, switching to a second line treatment with allopurinol should be considered, taking into account any comorbidities or preferences.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
In randomised controlled trials of patients with hyperuricaemia or gout, febuxostat reduced serum urate level more effectively than allopurinol.[113]Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461. https://www.nejm.org/doi/full/10.1056/NEJMoa050373 http://www.ncbi.nlm.nih.gov/pubmed/16339094?tool=bestpractice.com [114]Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548. https://onlinelibrary.wiley.com/doi/full/10.1002/art.24209 http://www.ncbi.nlm.nih.gov/pubmed/18975369?tool=bestpractice.com Open-label data suggest that this benefit is maintained for up to 40 months.[126]Becker MA, Schumacher HR, MacDonald PA, et al. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol. 2009;36:1273-1282. http://www.ncbi.nlm.nih.gov/pubmed/19286847?tool=bestpractice.com In these studies, however, allopurinol was administered at doses less than the maximum approved dose for this indication. A subsequent double blind non-inferiority trial demonstrated comparative efficacy for febuxostat and allopurinol in controlling flares when given at standard doses at 72 weeks.[116]O'Dell JR, Brophy MT, Pillinger MH, et al. Comparative effectiveness of allopurinol and febuxostat in gout management. NEJM Evid. 2022 Mar;1(3):10.1056/evidoa2100028. https://www.doi.org/10.1056/evidoa2100028 http://www.ncbi.nlm.nih.gov/pubmed/35434725?tool=bestpractice.com
Commonly reported adverse effects of febuxostat therapy include elevated liver function tests, headache, hypertension, diarrhoea, and arthralgia/stiffness.[127]Ernst ME, Fravel MA. Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout. Clin Ther. 2009;31:2503-2518. http://www.ncbi.nlm.nih.gov/pubmed/20109996?tool=bestpractice.com [128]Stevenson M, Pandor A. Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal. Health Technol Assess. 2009;13(Suppl 3):37-42. http://www.ncbi.nlm.nih.gov/pubmed/19846027?tool=bestpractice.com
Cardiovascular death and all-cause mortality were significantly more common among patients taking febuxostat than those taking allopurinol (4.3% vs. 3.2%, hazard ratio [HR] 1.34 [95% CI 1.03 to 1.73]; 7.8% vs. 6.4%, HR 1.22 [95% CI 1.01 to 1.47], respectively) in a multi-centre non-inferiority trial of patients with gout and cardiovascular disease.[119]White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018 Mar 12;378(13):1200-10. https://www.nejm.org/doi/10.1056/NEJMoa1710895 http://www.ncbi.nlm.nih.gov/pubmed/29527974?tool=bestpractice.com Febuxostat was non-inferior to allopurinol with respect to a composite primary outcome of cardiovascular events.
Conversely, evidence from subsequent systematic reviews suggest that there is no significant association with high blood pressure, all cause mortality, myocardial infarction, or stroke for either febuxostat or allopurinol, and no significant difference between the two treatments for the composite outcome of major adverse cardiovascular events (including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and unstable angina with urgent coronary revascularisation) among adult patients with hyperuricaemia and/or gout.[120]Barrientos-Regala M, Macabeo RA, Ramirez-Ragasa R, et al. The association of febuxostat compared with allopurinol on blood pressure and major adverse cardiac events among adult patients with hyperuricemia: a meta-analysis. J Cardiovasc Pharmacol. 2020 Oct;76(4):461-71. http://www.ncbi.nlm.nih.gov/pubmed/32675751?tool=bestpractice.com [121]Zhao L, Cao L, Zhao TY, et al. Cardiovascular events in hyperuricemia population and a cardiovascular benefit-risk assessment of urate-lowering therapies: a systematic review and meta-analysis. Chin Med J (Engl). 2020 Apr 20;133(8):982-93. https://www.doi.org/10.1097/CM9.0000000000000682 http://www.ncbi.nlm.nih.gov/pubmed/32106120?tool=bestpractice.com [122]Hay CA, Prior JA, Belcher J, et al. Mortality in patients with gout treated with allopurinol: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2021 Jul;73(7):1049-54. https://www.doi.org/10.1002/acr.24205 http://www.ncbi.nlm.nih.gov/pubmed/32286732?tool=bestpractice.com
Febuxostat should not be started during an acute attack, as it might prolong the attack or precipitate more attacks.[129]National Institute for Health and Care Excellence. Febuxostat for the management of hyperuricaemia in people with gout. Dec 2008 [internet publication]. https://www.nice.org.uk/guidance/ta164
Goal is to reduce uric acid level <360 micromol/L (<6 mg/dL) to prevent supersaturation and crystal formation.
Adjust dose according to serum urate target level of 360 micromol/L (6 mg/dL).
Primary options
febuxostat: 40-80 mg orally once daily
suppressive therapy
Treatment recommended for ALL patients in selected patient group
Suppressive (prophylactic) therapy such as non-steroidal anti-inflammatory drugs (NSAIDs), low-dose colchicine, or low dose oral corticosteroid should be considered during initiation and tapering of a urate-lowering agent.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [84]Qaseem A, Harris RP, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58-68. https://www.acpjournals.org/doi/full/10.7326/M16-0570 http://www.ncbi.nlm.nih.gov/pubmed/27802508?tool=bestpractice.com
The therapy should be continued for at least 3 to 6 months after reaching the target level of serum uric acid.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Prednisolone may be considered if both NSAIDs and colchicine are contraindicated, at the lowest dose to prevent gout flares.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Primary options
naproxen: 250 mg orally twice daily
OR
ibuprofen: 400 mg orally three times daily
OR
diclofenac potassium: 50 mg orally (immediate-release) two to three times daily
OR
meloxicam: 7.5 to 15 mg orally once daily
OR
indometacin: 25 mg orally three times daily
OR
celecoxib: 100-200 mg orally once daily
OR
colchicine: 0.6 mg orally once daily
Secondary options
prednisolone: 7.5 to 10 mg orally once daily initially, adjust to lowest dose that prevents gout flares
probenecid or sulfinpyrazone
If the patient cannot tolerate allopurinol or febuxostat, probenecid or sulfinpyrazone (uricosuric drugs) may be considered.[123]Hui M, Carr A, Cameron S, et al; British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2017 Jul 1;56(7):e1-20. [Erratum in: Rheumatology (Oxford). 2017 Jul 1;56(7):1246.] https://academic.oup.com/rheumatology/article/56/7/e1/3855179 http://www.ncbi.nlm.nih.gov/pubmed/28549177?tool=bestpractice.com
Both probenecid and sulfinpyrazone increase the renal excretion of uric acid. They are contraindicated in known over-producers of uric acid.
A 24-hour urine collection for uric acid should be obtained before prescribing probenecid or sulfinpyrazone.
If uric acid excretion exceeds 800 mg in 24 hours, probenecid and sulfinpyrazone are contraindicated, as they increase the risk of urate nephrolithiasis.
Probenecid and sulfinpyrazone are not effective in patients with renal insufficiency.
Primary options
probenecid: 250-1000 mg orally twice daily
OR
sulfinpyrazone: 100-400 mg orally twice daily
suppressive therapy
Treatment recommended for ALL patients in selected patient group
Suppressive (prophylactic) therapy such as non-steroidal anti-inflammatory drugs (NSAIDs), low-dose colchicine, or low dose oral corticosteroid should be considered during initiation and tapering of a urate-lowering agent.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [84]Qaseem A, Harris RP, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58-68. https://www.acpjournals.org/doi/full/10.7326/M16-0570 http://www.ncbi.nlm.nih.gov/pubmed/27802508?tool=bestpractice.com
The therapy should be continued for 3 to 6 months after reaching the target level of serum uric acid.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Prednisolone may be considered if both NSAIDs and colchicine are contraindicated, at the lowest dose to prevent gout flares.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Primary options
naproxen: 250 mg orally twice daily
OR
ibuprofen: 400 mg orally three times daily
OR
diclofenac potassium: 50 mg orally (immediate-release) two to three times daily
OR
meloxicam: 7.5 to 15 mg orally once daily
OR
indometacin: 25 mg orally three times daily
OR
celecoxib: 100-200 mg orally once daily
OR
colchicine: 0.6 mg orally once daily
Secondary options
prednisolone: 7.5 to 10 mg orally once daily initially, adjust to lowest dose to prevent gout flares
pegloticase
Intravenous pegloticase (a pegylated recombinant mammalian uricase) is an option for patients with gout who fail to achieve a uric acid level of <360 micromol/L (<6 mg/dL), and who continue to have frequent gout flares (≥2 flares/year) or have non-resolving subcutaneous tophi with conventional urate-lowering agents.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [124]Sundy JS, Baraf HS, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720. http://jama.jamanetwork.com/article.aspx?articleid=1104232 http://www.ncbi.nlm.nih.gov/pubmed/21846852?tool=bestpractice.com [125]Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021 Aug 11;8:CD010069. https://www.doi.org/10.1002/14651858.CD010069.pub3 http://www.ncbi.nlm.nih.gov/pubmed/34379791?tool=bestpractice.com
Gout flares may occur during initial treatment.
Pegloticase is associated with anaphylaxis and serious hypersensitivity reactions. Only administer this drug in a specialised care setting under a clinician who is experienced in managing infusion reactions and anaphylaxis. Monitor serum uric acid levels prior to each infusion. Discontinue pegloticase if serum uric acid level increases to >360 micromol/L (>6 mg/dL), particularly if two consecutive levels are >360 micromol/L (>6 mg/dL); patients who have lost therapeutic response are at an increased risk of developing infusion reactions or anaphylaxis. Other urate-lowering drugs should not be taken concomitantly as they affect serum uric acid levels. Pre-medicate patients with an antihistamine and corticosteroid prior to administration. Monitor patients closely during and after infusion.
Primary options
pegloticase: 8 mg intravenously every 2 weeks
suppressive therapy
Treatment recommended for ALL patients in selected patient group
Suppressive (prophylactic) therapy such as non-steroidal anti-inflammatory drugs (NSAIDs), low-dose colchicine, or a low-dose corticosteroid should be considered during initiation and tapering of a urate-lowering agent.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219 [81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com [84]Qaseem A, Harris RP, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58-68. https://www.acpjournals.org/doi/full/10.7326/M16-0570 http://www.ncbi.nlm.nih.gov/pubmed/27802508?tool=bestpractice.com
The therapy should be continued for 3 to 6 months after reaching the target level of serum uric acid.[81]FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. http://www.ncbi.nlm.nih.gov/pubmed/32391934?tool=bestpractice.com
Prednisolone may be considered if both NSAIDs and colchicine are contraindicated, at the lowest dose to prevent gout flares.[53]National Institute for Health and Care Excellence. Gout: diagnosis and management. Jun 2022 [internet publication]. https://www.nice.org.uk/guidance/ng219
Primary options
naproxen: 250 mg orally twice daily
OR
ibuprofen: 400 mg orally three times daily
OR
diclofenac potassium: 50 mg orally (immediate-release) two to three times daily
OR
meloxicam: 7.5 to 15 mg orally once daily
OR
indometacin: 25 mg orally three times daily
OR
celecoxib: 100-200 mg orally once daily
OR
colchicine: 0.6 mg orally once daily
Secondary options
prednisolone: 7.5 to 10 mg orally once daily initially, adjust to lowest dose to prevent gout flares

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer