Approach
The management of hypotonic hyponatraemia depends primarily on whether the onset is acute (i.e., <48 hours) or chronic (≥48 hours). This is because acute onset indicates the likelihood of cerebral oedema, which requires prompt treatment in a hospital. Chronic hyponatraemia should be managed more conservatively to avoid the consequences of rapid correction (e.g., myelinolysis); specific treatment depends on the volume status of the patient. Note that there is an increased risk of myelinolysis syndrome when hypokalaemia, liver disease or alcoholism is also present.[46][47]
Acute onset (<48 hours) or symptomatic
Cerebral oedema occurs more frequently when hyponatraemia develops acutely (i.e., <48 hours). In patients with cerebral oedema, symptoms are usually severe (e.g., nausea/vomiting, altered mental status, somnolence, seizures, coma).[2]
Supportive care (e.g., establishing intravenous access, giving supplemental oxygen, seizure control, intubation) should be initiated depending on the presentation.[3] Avoid giving hypotonic fluids as this can worsen cerebral oedema.
All patients with acute onset of hyponatraemia and/or symptoms should be treated promptly with hypertonic 3% saline.[2][3] The usual regimen is 100-300 mL of hypertonic 3% saline (or 2-4 mL/kg) given in 100 mL increments over 10 minutes (usually as a bolus infusion) until symptom improvement occurs. Evidence suggests that a ‘rapid intermittent bolus’ regime of fluid administration may be more effective at achieving the desired hourly reduction in sodium levels than a slow continuous infusion.[48] Once desired serum sodium concentration is achieved, hypertonic 3% saline can be stopped and fluid repletion continued with a suitable fluid according to clinical conditions and to correct any ongoing losses.[5]
Recommendations regarding the rate of serum sodium correction vary, with some sources recommending a rate of 3-6 mmol/L/day, while others recommend a more aggressive rate of 8-12 mmol/L/day (in the first 24 hours).[2][49][50] A rate of 1-2 mmol/L/hour can be used for several hours if symptoms are severe.[24] A rate of <8 mmol/L/day is recommended to prevent myelinolysis.[24][Figure caption and citation for the preceding image starts]: Brain magnetic resonance imaging of a patient with central pontine myelinolysis showing hypointensity within the basis pontisBMJ Case Reports 2013; doi:10.1136/bcr-2013-009970 [Citation ends].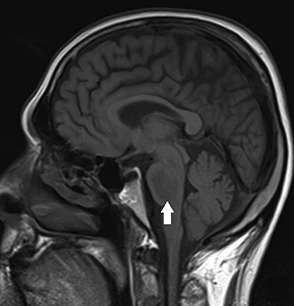 A more conservative approach to sodium correction is recommended in patients who have mild symptoms or who are asymptomatic. It should be noted that it may take some time for patients to recover from severe symptoms, and that symptoms may be difficult to assess if a patient is sedated or intubated.
A more conservative approach to sodium correction is recommended in patients who have mild symptoms or who are asymptomatic. It should be noted that it may take some time for patients to recover from severe symptoms, and that symptoms may be difficult to assess if a patient is sedated or intubated.
Serum sodium concentration should be tested every 2 hours to guide therapy until stabilisation occurs and hypertonic saline is no longer required. It should then be tested every 4-6 hours for 24 hours, and every 24 hours thereafter to monitor the ongoing rate of correction.[3] Urine output should also be monitored as spontaneous diuresis can occur, which may lead to overcorrection of the serum sodium concentration. If urine output changes abruptly, serum sodium levels should be reassessed. A urine output of <2400 mL/24 hours (or 100 mL/hour) has been proposed as a safe upper limit during hyponatraemia correction, but this has yet to be validated.[51] Patients should be managed in an environment where close clinical monitoring is available.[3]
Once the patient is stabilised, the underlying cause should be addressed, although urine studies should be obtained as early as possible as they may be affected by the administration of intravenous fluids. Patients who do not improve may require further diagnostic assessment.
Chronic onset (≥48 hours)
Patients with an onset of hyponatraemia ≥48 hours are usually asymptomatic or may have only mild symptoms. The goal in these patients is to normalise the serum sodium concentration gradually to prevent complications such as myelinolysis. Serum sodium concentration should be tested every 8-12 hours for 24 hours, and every 24 hours thereafter.
Specific treatment depends on the volume status of the patient and the underlying cause.
Hypovolaemic hyponatraemia:
Isotonic intravenous fluids (e.g., normal saline 0.9% or a balanced solution such as lactated Ringer's solution) should be administered in 250-1000 mL boluses to maintain blood pressure. Boluses can be repeated as necessary, and then followed by an infusion of 0.5 to 1 mL/kg/hour to replete intravascular volume until signs and symptoms are no longer present.[2] Rate of correction should follow the same principles as for acute onset. It is important that hypotonic fluids be avoided in these patients.
Serum sodium levels should be reassessed after repletion of volume as a concomitant disorder that is causing hyponatraemia may be present.
Underlying cause should be treated (e.g., treating severe nausea/vomiting, stopping diuretics if possible, treating mineralocorticoid deficiency).
Hypervolaemic or euvolaemic hyponatraemia:
All patients should be placed on fluid restriction (including all oral and intravenous fluids) of 1 L/day.[2][3] This should be adjusted based on measured urine output and set at 500 mL less than daily urine volume (e.g., a patient producing 1200 mL/day of urine should be placed on 700 mL/day fluid restriction). This conservative approach should increase the serum sodium concentration gradually (i.e., <8 mmol/L/day) and helps to prevent myelinolysis, which can occur if sodium levels are corrected too quickly.
Underlying cause should be treated. For patients with hypervolaemic hyponatraemia, causes include acute kidney injury/chronic kidney disease, congestive heart failure, cirrhosis, and nephrotic syndrome. For patients with euvolaemic hyponatraemia, causes include causative medications and excess fluid intake (e.g., exercise, surgery, primary polydipsia, potomania). The syndrome of inappropriate antidiuretic hormone (SIADH) is characterised by hypotonic hyponatraemia, concentrated urine, and a euvolaemic state.
A diuretic can be added if hypervolaemia is present and the underlying condition warrants it.[3] Choice of agent depends on the underlying condition. Loop diuretics are recommended in patients with congestive heart failure or nephrotic syndrome, while spironolactone (an aldosterone receptor antagonist) is recommended in patients with cirrhosis and ascites. The rate of correction of serum sodium should be closely monitored when using these agents and kept to <8 mmol/L/day to prevent myelinolysis.
If fluid restriction fails, a vasopressin receptor antagonist (also known as a vaptan) should be considered a second-line option in patients with SIADH or congestive heart failure.[3] Failure of fluid restriction can be predicted if at least one of the following is present:[1]
Urine osmolality >500 mmol/kg
Urine sodium (plus potassium) concentration is greater than the serum sodium concentration
Urine output <1500 mL/day
If on fluid restriction of <1 L/day the serum sodium concentration increase is <2 mmol/L over 24-48 hours
If the patient has excessive thirst
Vaptans directly block the action of vasopressin on the kidneys, thereby reducing water retention. They have been shown to be safe and effective at slowly correcting serum sodium levels; however, they have not been shown to be associated with mortality benefits when used for the long-term treatment of congestive heart failure or euvolaemic/hypervolaemic hyponatraemia.[52][53] [
 ]
There are also concerns that these drugs are associated with liver dysfunction. Once a vaptan is started, fluid restriction is lifted in order to prevent overcorrection.[3][54] One systematic review found that treatment with a vaptan increased the risk of overcorrection by approximately 3% compared with placebo.[53]
]
There are also concerns that these drugs are associated with liver dysfunction. Once a vaptan is started, fluid restriction is lifted in order to prevent overcorrection.[3][54] One systematic review found that treatment with a vaptan increased the risk of overcorrection by approximately 3% compared with placebo.[53]Oral urea is supported as an alternative to vaptans in European and US hyponatraemia guidelines, although evidence from large multicentre randomised trials is lacking. In one study of 58 hospitalised patients with hyponatraemia, oral urea increased sodium levels from 124 to 131 mmol/L. The treatment is generally well tolerated and may be used in outpatient settings.[2][9][55][56][57]
Overcorrection of serum sodium concentration
If an overcorrection of more than 8-12 mmol/L/day of serum sodium concentration occurs, then prompt intervention is required to lower the serum sodium concentration.[2] All active treatment should be stopped and treatment with free water intake and/or desmopressin should be initiated.
The Adrogue-Madias formula can be used to determine the sodium-lowering effect of 1 litre of fluid (e.g., dextrose 5% in water):
Change in serum sodium concentration (per 1 L) = (infusate sodium concentration - serum sodium concentration)/(total body water + 1)
where serum sodium concentration is in mmol/L and total body water is in litres. The sodium concentration of the infusate fluid must be known in order to use this formula, and the equation fails to take ongoing water and electrolyte losses through urine and stool into account.[2][24]
Total body water in the equation above can be estimated by using the following simple calculations:
0.5 x body weight in kilograms (for women and elderly men)
0.6 x body weight in kilograms (for younger men or children)
Sodium concentration of common fluids (per litre):
Normal saline (0.9%): 154 mmol/L
Lactated Ringer's solution: 130 mmol/L
Dextrose 5% in water: 0 mmol/L
Enteral water: 0 mmol/L
This formula may be inadequate at predicting serum sodium levels in individual patients. For this reason, some physicians recommend paying careful attention to the initial changes in the serum sodium level and starting with 50% to 75% of the calculated infusion rate, and then adjusting the rate according to the serum sodium level.
In some instances, a patient who is retaining water (e.g., medication-associated vasopressin release) may develop a water diuresis as the medication level drops, which may lead to overcorrection.
Electrolyte-free water excretion
Electrolyte-free water excretion (also known as electrolyte-free water clearance) may be calculated in patients with hypervolaemic or euvolaemic hyponatraemia. It is particularly useful in patients who are not responding to general fluid restriction measures, and helps to determine ongoing urinary water retention or excretion and the amount of free water restriction required to correct the serum sodium concentration.
The following formula can be used:
Electrolyte-free water excretion = V × [1 - (UNa + UK)/(PNa)]
Where V is the urine flow rate, UNa is the urine concentration of sodium (mmol/L), UK is the urine concentration of potassium (mmol/L), and PNa is the plasma concentration of sodium (mmol/L).
If the value is positive, free water intake should be restricted to less than the electrolyte-free water excretion value, if feasible, in order to induce a negative free water balance. If the value is very low, this may be difficult to achieve. If the value is negative, free water restriction will not be effective and alternative treatment options (e.g., vaptans) should be considered.[43][44]
Use of this content is subject to our disclaimer