History and exam
Key diagnostic factors
common
presence of risk factors
Includes history of exposure to Triatoma species; history of blood transfusion; history of organ transplantation; history of immunosuppression; healthcare or laboratory occupations; poverty; low education levels; travel to endemic areas; residence in endemic or high-risk areas; ingestion of contaminated food or drink; and positive family history (including mother with Chagas disease).
prolonged fever
May be present in acute-phase disease. A non-specific sign, characterised by prolonged (7-30 days) and constant febrile temperatures (usually 37.5°C to 38.5°C [99.5°F to 101°F]) with nocturnal elevation.
In some cases of ingestion of contaminated food or drink, cases may have a short course with fever (usually <7 days).
palpitations
In the acute phase, this may be a sign of acute myocarditis.
syncope or presyncope
hepatosplenomegaly
Mild or moderate. Typically painless.
enlarged lymph nodes
Mild or moderate. Typically painless. Principal regions: auricular, cervical, sub-mandibular, axillary, and inguinal.
tachycardia
May be present in acute-phase disease as a sign of acute myocarditis.
hypotension
May be present in acute-phase disease as a sign of acute myocarditis.
cardiomegaly
May be present in acute-phase disease as a sign of acute myocarditis or pericardial effusion.
May be present in chronic-phase disease as a sign of chronic heart failure, and is generally associated with systolic dysfunction.
uncommon
dysphagia
In the chronic phase, dysphagia for liquids and solids may be associated with gastrointestinal involvement.
regurgitation/aspiration
In the chronic phase, this may be associated with gastrointestinal involvement.
odynophagia
In the chronic phase, this may be associated with gastrointestinal involvement.
substernal discomfort
In the chronic phase, this may be associated with gastrointestinal involvement.
prolonged constipation
In the chronic phase, this may be associated with gastrointestinal involvement. Indicates intestinal occlusion, or sigmoid volvulus.
acute abdominal pain
In the chronic phase, this may be associated with gastrointestinal involvement. This can be a gastrointestinal emergency (bowel ischaemia or volvulus). May also be associated with congestive hepatopathy in chronic-phase disease with cardiac involvement.
abdominal distension
In the chronic phase, gaseous or asymmetrical distension may be associated with gastrointestinal involvement. Sign of megacolon, intestinal occlusion, or sigmoid volvulus.
swelling around the site of inoculation
Specific evidence of acute-phase disease. Related to vectorial transmission. Usually called inoculation chagoma. Represents an area where the parasite entered the skin or mucous membrane.
Romaña's sign (ophthalmoganglionar complex) occurs when the inoculation site is the conjunctiva, with unilateral periocular oedema. This sign is associated with subcutaneous inflammatory nodule or non-purulent unilateral palpebral oedema, and conjunctivitis with ipsilateral regional preauricular lymphadenopathy.
Chagoma and Romaña's sign are pathognomonic, but only occur in a minority of patients.[2][Figure caption and citation for the preceding image starts]: Child with an inoculation chagoma (Romaña's sign)Grupo de Estudo em Correlalacao Anatomo-Clinica, Clínica Médica, Pontificia Universidade Catolica de Campinas, Sao Paulo, Brazil; used with permission [Citation ends].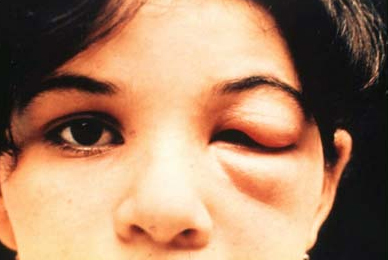
jaundice
May be present in acute-phase disease after ingestion of contaminated food or drink.
abdominal rebound tenderness
May be present in chronic-phase disease. Signals presence of a gastrointestinal emergency such as bowel ischaemia or volvulus.
clinical evidence of meningeal irritation
Occurs in cases of meningoencephalitis (in acute phase, neonates, or reactivation).
clinical signs of a cerebral mass lesion
Occurs in cases of meningoencephalitis (in acute phase, neonates, or reactivation), and is associated with cardioembolic stroke in patients with chronic-phase disease and cardiac involvement.
Other diagnostic factors
common
irritability
May be present in children with acute-phase disease.
anorexia or fatigue
May be present in acute-phase disease.
vomiting or diarrhoea
May be present in acute-phase disease.
headache
May be present in acute-phase disease.
myalgia
May be present in acute-phase disease.
reduced exercise tolerance
May be a symptom of congestive heart failure following Chagas-induced cardiac disease.
dizziness
In the chronic phase, this may be a sign of cardiopathy (conduction system disease, or arrhythmias).
thromboembolic phenomena (e.g., stroke, pulmonary embolism)
May be present in chronic-phase disease as a sign of cardiopathy.
uncommon
dyspnoea
May be a symptom of congestive heart failure following Chagas-induced cardiac disease.
cough
May be present in acute-phase disease as a sign of acute myocarditis.
generalised oedema
May be present as a sign of congestive heart failure.
pericarditis
May be present in acute-phase disease as a sign of acute myocarditis.
epigastric pain and/or haematemesis
In the acute phase, this may be associated with ingestion of contaminated food or drink.
melaena or haematochezia
In the acute phase, this may be associated with ingestion of contaminated food or drink.
rash
May be present in acute-phase disease. A non-specific sign characterised by rash with variable localisation, with or without pruritus.
seizures or tremors
May occur with acute-phase meningoencephalitis. Indicates a poor prognosis.
Risk factors
strong
living in endemic area
Chagas disease is endemic in 21 Latin American countries and it is estimated that 6-7 million people worldwide are infected with Trypanosoma cruzi, including 300,000 people residing in the US and 80,000 in Spain.[11] WHO: Chagas disease (American trypanosomiasis) Opens in new window Infected residents have also been reported in Switzerland, France, Italy, Canada, Australia, and Japan.[2]
exposure to Triatoma species
Triatomines hide in the nests or resting places of wild animals. They feed on blood while the animal is sleeping (sylvatic cycle). Human activities sometimes may expose them to these insects. Some of these insect species have adapted to human dwellings where they hide in crevices, emerging at night for their blood meal (domestic cycle). The distribution of animal reservoirs (sylvatic or domesticated) in different habitats allows for evaluation of the probable place of transmission, indicating potential risk for vectorial and oral transmission.[12][42][43][73]
Classical transmission occurs by vectors that hide inside cracks in mud or adobe houses.[74] Studies have shown that vector populations are still abundant and highly prevalent in poor rural housing.[75][76] Heterogeneous habitat conditions are expected to affect triatomine population parameters, dispersal, control, and infection with Trypanosoma cruzi. The presence of domestic animals increases colonisation of houses and makes control more difficult.[77][78]
low socio-economic status
Chagas disease is a preventable condition that affects mostly low-income populations or those previously living in rural areas of endemic regions. Like many other parasitic infections, it is classically associated with poverty and low educational level in both endemic and non-endemic areas.[31][77][79][80]
consumption of contaminated food or drink
Accidental ingestion of triatome faeces or triatomine structures can occur with unhygienic food preparation. Some marsupial species (Didelphis species) can harbour and excrete Trypanosoma cruzi in their anal glands, leading to contamination of food and/or utensils. Some fruits (e.g., açaí, juçara, bacaba, and sugar cane) are commonly contaminated as vectors and sylvatic animals share this habitat. People become infected after drinking these juices.[9]
blood transfusion
In most endemic Latin American countries, blood donors are routinely screened for Chagas disease.[27][81] Massive migration from Latin America to US cities has, however, increased the number of infected individuals in the US.[82] The first reported case of transfusion-related transmission in the US was in 1987, but US screening for Trypanosoma cruzi in donated blood was not widely practised until 2007.[83] At least 2300 infected blood donors have been reported by blood banks across the US as of December 2017.[11] The risk of transmission varies between 12% and 44% for a single transfusion of 500 mL of infected blood.[84][85] Risk depends on multiple factors, such as the degree of parasitaemia in the donor, the type of blood component transfused, and the parasite strain.[83][86][87]
organ transplantation
Transplantation places the recipient at an additional risk for Chagas disease, due to induced immunosuppression. Infection has been described after heart, kidney, bone marrow, or liver transplantation.[21][88][89][90][91][92][93] In addition, acute disease may occur after bone marrow transplantation.[15][94][95][96][97][98]
history of immunosuppression
Patients with immunosuppression (acquired or induced), in association with chronic Chagas disease, may develop a typical syndrome of reactivation.
climate change
Global climate changes are expected to affect populations of triatomines living in the surroundings of domestic dwellings much more than domestic bug populations.[78][99][100][101] This will have an impact on the ecosystems that influence the dynamics of the sylvatic cycle: if their ecosystems are destroyed, the (sylvatic) insect vectors will search for alternative blood sources, and will consequently adapt to new environments close to human dwellings.[100]
weak
laboratory work occupations
In laboratories, infection has been described via contaminated needles, exposure to the faeces of triatomine bugs, handling of infectious cultures, and possibly by inhalation.[104] The rate of recognised laboratory accidents per high-risk person-year has been estimated as 1 accident in 15 person-years, and infections per high-risk person-year as one infection per 46 person-years.[55] Depending on the nature of the accident, the individual risk may range from weak to strong.
travel to endemic areas
Travel to rural areas in endemic regions poses an extremely low risk for acquisition of Chagas disease.[84][105][106][107][108][109][110][111] No cases of acquired infection during travel have been documented, however, travellers could be at risk if staying in poor-quality housing in recognised endemic areas.[84]
mother with Chagas disease
Vertical (mother-to-child) transmission occurs mainly in the third month of pregnancy. The risk of transmission from infected mother to child ranges from 0% to 8%.[41][112][113][114][115][116][117] Due to human migration, vertical transmission may also occur in non-endemic areas. Evidence suggests that approximately 0.3% of pregnant Latina women in Houston, Texas are seropositive.[118]
Use of this content is subject to our disclaimer