Aetiology
Trypanosoma cruzi has sylvatic (occurring in wild animals), peridomestic, and domestic cycles. The domestic cycle is maintained by triatomines adapted to human dwellings, transmitting the parasite from domestic animals to humans and between humans.[12][42][43] The sylvatic cycle is maintained by triatomines and wild animals. In the peridomestic cycle, the infection is maintained among domestic animals in areas surrounding human dwellings, by peridomestic triatomines (and occasionally through exchange with the sylvatic cycle, such as dogs and cats hunting wild animals, or sylvatic animals invading areas surrounding human dwellings).[44][45][46][47] Birds and cold-blooded animals are resistant to infection.
The classic mode of transmission of T cruzi is by contact with the faeces or urine of blood-sucking triatomine bugs (commonly known as kissing bugs in the US), which typically attack at night.[29][48] More than 150 triatomine species have been described in endemic areas, and include Alberprosenia species; Belminus species; Eratyrus species; Microtriatoma species; Panstrongylus species; Psammolestes species; Rhodnius species; andTriatoma species.[42][49][50][51] The principal vector in the US is T. sanguisuga.[52][53] There are very few reports of transmission by vectors in humans in the US; however, increased domestic vector presence, globalisation, and potential future rises in temperature have raised awareness that vector-borne transmission may become established in the US.[39][54]
Other primary modes of transmission include blood transfusions, consumption of contaminated food or drink, and vertical transmission (causing congenital disease). Secondary modes of transmission include organ transplantation, laboratory accidents, handling infected animals, sexual activity (via wounds, sperm, or menstrual fluids), and criminally induced infection (inoculation or oral).[9][42][55][56] It is important to note that sexual transmission is possible in the case of acute Chagas disease due to the high level of parasitaemia but, unlike the other mechanisms, this pathway does not occur in the context of chronic disease.
An increased number of cases and micro-epidemics of oral transmission have been observed in Latin American countries (mainly in the Amazon region of Brazil). Several outbreaks of acute Chagas disease involving groups of people, often families, have been reported from endemic areas. Most commonly, oral ingestion occurs via home-made açaí, other palm products, and sugar cane juice. However, it may also occur after ingestion of water contaminated with fruit juice and after eating the raw meat of sylvatic animals.[9]
[Figure caption and citation for the preceding image starts]: Triatoma sanguisuga: vector species with wide distribution in the US Cleber Galvao, PhD, Laboratório Nacional e Internacional de Referência em Taxonomia de Triatomíneos, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil; used with permission [Citation ends].
Pathophysiology
The parasite is classically transmitted by an infected triatomine bug. Triatomines hide in the nests or resting places of wild animals and feed on blood while the animal is sleeping (sylvatic cycle). Some of these insect species have adapted to human dwellings where they hide in crevices, emerging at night for their blood meal (domestic cycle). Within the vector's intestine, Trypanosoma cruzi undergoes several successive developmental stages, the last of which is a flagellated form living in the vector's rectum. Ingestion of the blood meal causes the vector to defecate and deposit faeces containing infectious metacyclic trypomastigotes onto the victim's skin, close to the bite wound. Upon awakening, the victim commonly rubs the itching bite area, pushing the trypanosome-laden faeces into the bite wound or onto the conjunctiva. Metacyclic trypomastigotes enter the victim's bloodstream through the bite wound or penetrate mucous membranes such as the conjunctiva (leading to Romaña's sign). This initiates the acute stage of disease.[4][26][42] This infective form of the parasite invades macrophage cells and transforms into intracellular amastigotes. The amastigotes multiply by binary fission and are released as trypomastigotes into the bloodstream and tissues.[4][12][26]
The incubation period varies according to the mode of transmission. The incubation period after vectorial transmission is 4 to 15 days; after transfusional transmission, it is 30 days or more (up to 4 months); after ingestion of contaminated food or drink, 3 to 22 days; and after accidental transmission, up to 20 days. Vertical transmission can occur in any gestation period, or during delivery.[57][58][59][60][61]
Trypomastigotes infect new cells of various tissues (e.g., reticuloendothelial system, myocardium, muscles, nervous system) and transform into intracellular amastigotes. After infection, inflammatory responses, cellular lesions, and fibrosis occur sequentially (mainly in the heart, oesophagus, and colon). In the acute phase, multiple cycles of intracellular parasite multiplication occur. This leads to high parasitaemia which further amplifies inflammation and cell lesions although, this process is less intense during chronic Chagas disease.
Myocytes and nerve cells (causing autonomic denervation) are typically affected. Direct destruction occurs by intracellular parasitism, necrosis related to inflammation, and other cytotoxic mechanisms. Fibrosis in Chagas cardiomyopathy is more intense than the fibrosis associated with any other cardiac disease. Cardiac involvement in the chronic phase is due to destruction of the conduction system, myocytes, and parasympathetic cardiac nerves. In association with the appearance of arrhythmogenic electric foci in the inflammatory areas, it gives rise to arrhythmic syndrome.[4][62][63][64][65] The hypertrophy of myocytes, and the intense fibrosis replacing the destroyed cells, predispose to cardiac dilation and failure. The left ventricular wall thins, typically allowing for the formation of an apical aneurysm. Thrombi are often present in such aneurysms, easily explaining the common occurrence of systemic and pulmonary thromboembolism.[4][14][64]
Parasympathetic intramural denervation is irregularly found within the gastrointestinal system and mainly affects the oesophagus and colon (most frequently the sigmoid colon). The affected intestine may have a normal macroscopic appearance with functional peristaltic disturbance but may also dilate, leading to mega-oesophagus or megacolon. Volvulus of the sigmoid colon is a rare complication appearing in advanced cases, and is associated with a high risk of intestinal necrosis.[66][67][68][69][70]
The factors that predispose a patient in the indeterminate phase of T. cruzi infection to develop symptomatic disease are not defined. Many factors may contribute.[71][72] These include: exposure to T. cruzi reinfection in areas with sustained vector transmission; male sex; parasite load; host genetic factors; nutritional status; patients’ social context and quality of life; and presence of comorbidities (important in the pathogenesis of chronic symptomatic/determinate Chagas disease).[72]
[Figure caption and citation for the preceding image starts]: Life cycle of Trypanosoma cruzi, the causative parasite of Chagas diseaseCenters for Disease Control and Prevention, Atlanta, GA, USA: Public Health Image Library ID # 3384 (Alexander J. da Silva, PhD/Melanie Moser, 2002) [Citation ends].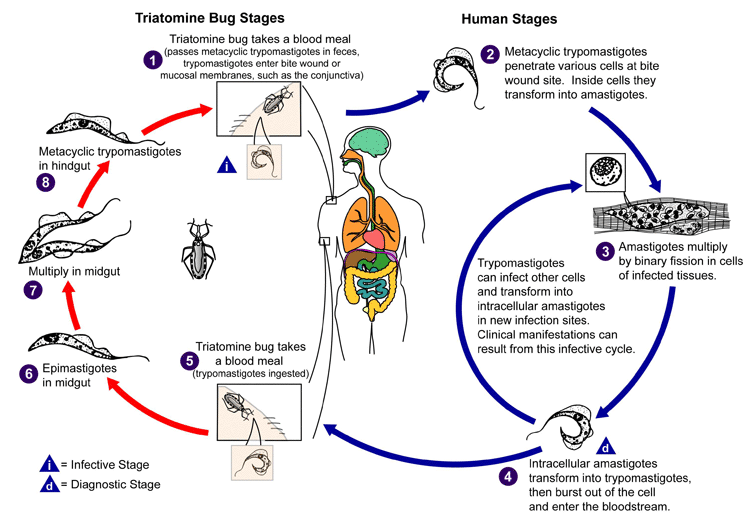
Classification
Clinical phases of infection
Acute phase
Usually lasts 3-8 weeks (up to 12 weeks in some cases), and is defined by evidence of Trypanosoma cruzi in the peripheral blood. Patients remain infected for life if not treated during this phase. Most patients have no symptoms, mild symptoms, or a non-specific febrile syndrome. Rarely, they may present with more severe symptoms such as myocarditis or meningoencephalitis.[9][10]
Chronic phase
Develops after many decades if suitable treatment is not given during the acute phase. Approximately 60% to 70% of infected individuals remain asymptomatic throughout their lifetime, while 20% to 30% of patients develop symptomatic disease.[2][11] Asymptomatic disease progresses to chronic symptomatic disease at a rate of 1.85% to 7.00% annually.[2]
The various clinical forms of disease may occur separately or simultaneously:
Indeterminate form: the most common form. Patients can be asymptomatic for decades after the acute phase. While serology is positive for T. cruzi these patients have anatomically and physiologically normal x-ray results of the heart, oesophagus, and colon, and no abnormal changes on echocardiography.[12]
Cardiac form: occurs between the 2nd and 4th decades of life, typically 5-15 years after the initial infection, and affects up to 30% of patients.[4][13][14]
Gastrointestinal form: uncommon in countries north of the equator. Oesophagopathy affects 5% to 10% of patients, and colonopathy affects 3% to 5% of patients.[13]
Mixed form (cardiac and gastrointestinal).
Reactivation phase
Chronic disease may become acute in immunocompromised patients (e.g., AIDS, haematological cancers, post-organ transplantation, high-dose immunosuppressive therapy) due to T. cruzi reactivation.[15] Patients usually present with meningoencephalitis or myocarditis; however, dermatological manifestations may also occur.[8][16]
Chagas disease reactivation is part of the diagnostic criteria for AIDS in Brazil.[17][18]
Use of this content is subject to our disclaimer