Summary
Definition
History and exam
Key diagnostic factors
- presence of risk factors
- decreased peripheral vision
- night blindness
- impaired dark adaptation
- decreased central acuity
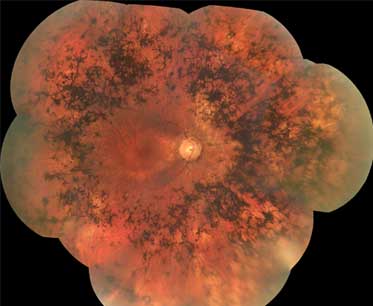
- atrophy of retinal pigment epithelium
- bone spicule pigmentation
Other diagnostic factors
- waxy pale optic nerve
- photopsias
- refractive error
- cataracts
- retinal vascular attenuation
- cystoid macular oedema
- vitreous cells
- glare from bright lights
- abnormal colour vision
- keratoconus
- glaucoma
- optic nerve head drusen
- Coats-like retinopathy
- Leber's congenital amaurosis
Diagnostic investigations
1st investigations to order
- assessment of visual acuity
- full field perimetry
- full field electroretinogram (ERG)
Investigations to consider
- elevated final dark-adapted threshold
- optical coherence tomography (OCT)
- genetic testing
- adaptive optics imaging
- wide-field fundus autofluorescence (FAF)
Treatment algorithm
Contributors
Authors
Mark E. Pennesi, MD, PhD

Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Disclosures
MEP serves on the scientific advisory board and executive committee for the Foundation Fighting Blindness.
Paul Yang, MD, PhD

Associate Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Disclosures
PY acted as a consultant for Applied Genetic Technologies Corp in 2019 and was paid for a meeting regarding XLRP gene therapy, for which there was no agreement to disseminate information.
Acknowledgements
Dr Mark E. Pennesi and Dr Paul Yang would like to gratefully acknowledge Dr Richard G. Weleber and Dr Peter J. Francis, previous contributors to this topic.
Disclosures
RGW has served as a consultant to Novartis, Pfizer, and Wellstat, is a member of the scientific advisory board for Applied Genetic Technologies Corp, and serves on the scientific advisory board for the Foundation Fighting Blindness (the relationship has been reviewed and managed by Oregon Health & Science University). RGW also reports having received grants and personal fees from the Foundation Fighting Blindness and Applied Genetic Technologies Corp, and other support from Sanofi-Fovea, all outside the submitted work. In addition, RGW has a patent (US patent 8,657,446, Method and apparatus for visual field monitoring, also known as Visual Field Monitoring and Analysis, or VFMA, which has not been issued). PJF declares that he has no competing interests.
Peer reviewers
Scott Fraser, MD, FRCS (Ed), FRCOphth
Consultant Ophthalmologist
Sunderland Eye Infirmary
Sunderland
UK
Disclosures
SF declares that he has no competing interests.
Elias Traboulsi, MD
Professor of Ophthalmology
Director
Center for Genetic Eye Diseases
Cole Eye Institute
Cleveland Clinic
Cleveland
OH
Disclosures
ET declares that he has no competing interests.
Use of this content is subject to our disclaimer