Coxiella burnetii infection is a notifiable disease in the US; however, reporting is not required in many other countries.
Most people infected with C burnetii remain asymptomatic. For those with symptoms, more than 30 different clinical syndromes have been described.
Diagnosis requires a high level of suspicion in a febrile patient who presents with non-specific symptoms. Recent contact with parturient animals is very rarely reported. Most laboratories do not culture C burnetii, because it is technically difficult. Culturing C burnetii requires a biosafety level 3 containment, because of the organism's significant infectivity and potential for use as a weapon of bioterrorism.[2]Hartzell JD, Wood-Morris RN, Martinez LJ, et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 May;83(5):574-9.
https://www.mayoclinicproceedings.org/article/S0025-6196(11)60733-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/18452690?tool=bestpractice.com
[52]Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Inf Dis. 2005 Apr;5(4):219-26.
http://www.ncbi.nlm.nih.gov/pubmed/15792739?tool=bestpractice.com
Therefore, diagnosis relies on serological analysis. Polymerase chain reaction (PCR) may be used for diagnosis of acute infection; however, it is not always readily available.
History
Recent animal contact and/or residency in or travel to endemic areas should be ascertained, but an exposure history is frequently lacking. Other risk factors include male gender and age 30 to 70 years. The clinician should enquire about pre-existing cardiac disease (e.g., history of rheumatic fever, bicuspid aortic valve, congenital heart disease, prosthetic heart valves, valve regurgitation, stenosis grade ≥II, or mitral valve prolapse), immunosuppression, vascular abnormalities, or pregnancy because these conditions predispose to the development of a persistent focalised infection.
Clinical presentation: acute infection
For most patients (up to 60%), infection is either asymptomatic or mild and self-limiting and spontaneously resolves within 2 weeks. Common presentations include fever, flu-like illness, pneumonia, and hepatitis.[1]Marrie TJ, Raoult D. Coxiella burnetii. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. 6th ed. Philadelphia, PA: Churchill Livingstone; 2005.[2]Hartzell JD, Wood-Morris RN, Martinez LJ, et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 May;83(5):574-9.
https://www.mayoclinicproceedings.org/article/S0025-6196(11)60733-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/18452690?tool=bestpractice.com
[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
[5]Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88.
http://www.ncbi.nlm.nih.gov/pubmed/16503466?tool=bestpractice.com
[38]Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999 Oct;12(4):518-53.
https://cmr.asm.org/content/12/4/518.full
http://www.ncbi.nlm.nih.gov/pubmed/10515901?tool=bestpractice.com
The classic presentation of acute infection is a flu-like illness that includes an abrupt onset of high fever (39°C to 40°C [102°F to 104°F]), chills, malaise, cough, headache, fatigue, and myalgia. The fever may be isolated and may last up to 14 days, but in untreated patients it may last up to 57 days.[38]Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999 Oct;12(4):518-53.
https://cmr.asm.org/content/12/4/518.full
http://www.ncbi.nlm.nih.gov/pubmed/10515901?tool=bestpractice.com
Patients may present with pneumonia that is usually mild with a cough (24% to 90% of patients) and sometimes pleuritic chest pain. Lung exam may reveal inspiratory crackles, rhonchi, or wheezing. Hepatitis is another frequent presentation of acute infection; jaundice is rare, but hepatomegaly may be palpable.[38]Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999 Oct;12(4):518-53.
https://cmr.asm.org/content/12/4/518.full
http://www.ncbi.nlm.nih.gov/pubmed/10515901?tool=bestpractice.com
Less common presentations, according to body system, include:
Cardiovascular: acute endocarditis, myocarditis, pericarditis, myopericarditis, arterial or venous thrombosis
Neurological: meningoencephalitis, meningitis
Cutaneous: maculopapular or purpuric rash, erythema nodosum
Haematological: thrombocytopenia, mononucleosic syndrome, haemophagocytosis, anaemia (haemolytic and transient hypoplastic), bone marrow necrosis, prolonged activated partial thromboplastin time (aPTT) with lupus anticoagulant (anti-prothrombinase activity)
Immune system: lymphadenitis
Muscular: rhabdomyolysis
Gastrointestinal: cholecystitis, gastroenteritis, pancreatitis, splenic rupture, mesenteric panniculitis
Genital: orchitis, epididymitis, priapism
Endocrine: thyroiditis, inappropriate secretion of antidiuretic hormone
Renal: glomerulonephritis.
Cutaneous manifestations (e.g., maculopapular or purpuric rash, erythema nodosum) may occur in up to 20% of cases during acute infection.[5]Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88.
http://www.ncbi.nlm.nih.gov/pubmed/16503466?tool=bestpractice.com
A purpuric rash may also occur in approximately 19% of patients with endocarditis following a chronic infection.[38]Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999 Oct;12(4):518-53.
https://cmr.asm.org/content/12/4/518.full
http://www.ncbi.nlm.nih.gov/pubmed/10515901?tool=bestpractice.com
People with meningoencephalitis may present with a severe headache, seizures, or coma.[53]Drancourt M, Raoult D, Xeridat B, et al. Q fever meningoencephalitis in five patients. Eur J Epidemiol. 1991 Mar;7(2):134-8.
http://www.ncbi.nlm.nih.gov/pubmed/2044709?tool=bestpractice.com
Encephalitic signs may include behavioural or psychiatric disturbances.[54]Bernit E, Pouget J, Janbon F, et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002 Mar 25;162(6):693-700.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/211336
http://www.ncbi.nlm.nih.gov/pubmed/11911724?tool=bestpractice.com
Acute endocarditis, arterial or venous thrombosis, thrombocytopenia, prolonged aPTT with lupus anticoagulant (antiprothrombinase activity), cholecystitis, and glomerulonephritis are autoimmune antiphospholipid-associated clinical presentations.[55]Ordi-Ros J, Selva-O'Callaghan A, Monegal-Ferran F, et al. Prevalence, significance, and specificity of antibodies to phospholipids in Q fever. Clin Infect Dis. 1994 Feb;18(2):213-8.
http://www.ncbi.nlm.nih.gov/pubmed/8161629?tool=bestpractice.com
[56]Newcombe JP, Gray PE, Palasanthiran P, et al. Q Fever with transient antiphospholipid antibodies associated with cholecystitis and splenic infarction. Pediatr Infect Dis J. 2013 Apr;32(4):415-6.
http://www.ncbi.nlm.nih.gov/pubmed/23271442?tool=bestpractice.com
[57]Million M, Thuny F, Bardin N, et al. Antiphospholipid antibody syndrome with valvular vegetations in acute Q fever. Clin Infect Dis. 2016 Mar 1;62(5):537-44.
http://www.ncbi.nlm.nih.gov/pubmed/26585519?tool=bestpractice.com
[58]Lee CH, Chuah SK, Pei SN, et al. Acute Q fever presenting as antiphospholipid syndrome, pneumonia, and acalculous cholecystitis and masquerading as Mycoplasma pneumoniae and hepatitis C viral infections. Jpn J Infect Dis. 2011;64(6):525-7.
http://www.ncbi.nlm.nih.gov/pubmed/22116335?tool=bestpractice.com
Chronic fatigue is being recognised as an increasingly important complication of acute C burnetii infection leading to long-term disability.[59]Wildman MJ, Smith EG, Groves J, et al. Chronic fatigue following infection by Coxiella burnetii (Q fever): ten-year follow-up of the 1989 UK outbreak cohort. QJM. 2002 Aug;95(8):527-38.
https://academic.oup.com/qjmed/article/95/8/527/1698439
http://www.ncbi.nlm.nih.gov/pubmed/12145392?tool=bestpractice.com
[60]Ayres JG, Wildman M, Groves J, et al. Long-term follow-up of patients from the 1989 Q fever outbreak: no evidence of excess cardiac disease in those with fatigue. QJM. 2002 Aug;95(8):539-46.
https://academic.oup.com/qjmed/article/95/8/539/1698443
http://www.ncbi.nlm.nih.gov/pubmed/12145393?tool=bestpractice.com
[61]Morroy G, Keijmel SP, Delsing CE, et al. Fatigue following acute Q-fever: a systematic literature review. PLoS One. 2016;11(5):e0155884.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0155884
http://www.ncbi.nlm.nih.gov/pubmed/27223465?tool=bestpractice.com
How this complication develops remains unclear. Antibiotics are not effective for managing chronic fatigue associated with C burnetii infection, but behavioural psychotherapy may help patients who develop this complication.[62]Keijmel SP, Delsing CE, Bleijenberg G, et al. Effectiveness of long-term doxycycline treatment and cognitive-behavioral therapy on fatigue severity in patients with Q fever fatigue syndrome (Qure Study): a randomized controlled trial. Clin Infect Dis. 2017 Apr 15;64(8):998-1005.
http://www.ncbi.nlm.nih.gov/pubmed/28329131?tool=bestpractice.com
Clinical presentation: persistent focalised infections
In a small proportion of patients, primary infection leads to persistent focalised infections (e.g., endocarditis, vascular infection, osteoarticular infection, lymphadenitis).[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
Persistent focalised infections can be diagnosed from 3 months to 17 years after the acute illness.[63]Wilson HG, Neilson GH, Galea EG, et al. Q fever endocarditis in Queensland. Circulation. 1976 Apr;53(4):680-4.
http://www.ncbi.nlm.nih.gov/pubmed/1253390?tool=bestpractice.com
Alternatively, they may occur with no history of acute illness.[5]Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88.
http://www.ncbi.nlm.nih.gov/pubmed/16503466?tool=bestpractice.com
Endocarditis is the most common form of persistent focalised infection and occurs in up to 70% of cases.[2]Hartzell JD, Wood-Morris RN, Martinez LJ, et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 May;83(5):574-9.
https://www.mayoclinicproceedings.org/article/S0025-6196(11)60733-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/18452690?tool=bestpractice.com
It usually develops in patients with underlying heart disease.[5]Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88.
http://www.ncbi.nlm.nih.gov/pubmed/16503466?tool=bestpractice.com
Peripheral manifestations of endocarditis are rarely present.[38]Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999 Oct;12(4):518-53.
https://cmr.asm.org/content/12/4/518.full
http://www.ncbi.nlm.nih.gov/pubmed/10515901?tool=bestpractice.com
For these patients, fever is often absent, and vegetations are usually absent or small. There may be augmentation of a known heart murmur, signs of heart failure, and arterial emboli. Vascular infections (e.g., aneurysm, vascular prosthetic infection) are also common.
Less common presentations include osteoarticular infections (e.g., culture-negative prosthetic joint infection, spondylodiscitis, osteomyelitis, osteoarthritis) and chronic lymphadenitis. Patients presenting with chronic lymphadenitis may be at risk of lymphoma.[64]Melenotte C, Million M, Audoly G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood. 2016 Jan 7;127(1):113-21.
http://www.ncbi.nlm.nih.gov/pubmed/26463422?tool=bestpractice.com
Rare presentations include chronic interstitial pneumonitis, lung pseudotumour, lung fibrosis, uveitis, optic neuritis, giant cell arteritis, or Takayasu's arteritis.[65]Million M, Halfon J, Le Lez ML, et al. Relapsing uveitis and optic neuritis due to chronic Q fever. Br J Ophthalmol. 2011 Jul;95(7):1026-7.
http://www.ncbi.nlm.nih.gov/pubmed/20733024?tool=bestpractice.com
[66]Odeh M, Oliven A. Temporal arteritis associated with acute Q fever. A case report. Angiology. 1994 Dec;45(12):1053-7.
http://www.ncbi.nlm.nih.gov/pubmed/7985833?tool=bestpractice.com
[67]Lefebvre M, Grossi O, Agard C, et al. Systemic immune presentations of Coxiella burnetii infection (Q fever). Semin Arthritis Rheum. 2010 Apr;39(5):405-9.
http://www.ncbi.nlm.nih.gov/pubmed/19110298?tool=bestpractice.com
[68]Baziaka F, Karaiskos I, Galani L, et al. Large vessel vasculitis in a patient with acute Q-fever: a case report. IDCases. 2014 Jul 31;1(3):56-9.
https://www.sciencedirect.com/science/article/pii/S2214250914000237
http://www.ncbi.nlm.nih.gov/pubmed/26952153?tool=bestpractice.com
Large vessel vasculitis is an autoimmune antiphospholipid-associated clinical presentation.[55]Ordi-Ros J, Selva-O'Callaghan A, Monegal-Ferran F, et al. Prevalence, significance, and specificity of antibodies to phospholipids in Q fever. Clin Infect Dis. 1994 Feb;18(2):213-8.
http://www.ncbi.nlm.nih.gov/pubmed/8161629?tool=bestpractice.com
[56]Newcombe JP, Gray PE, Palasanthiran P, et al. Q Fever with transient antiphospholipid antibodies associated with cholecystitis and splenic infarction. Pediatr Infect Dis J. 2013 Apr;32(4):415-6.
http://www.ncbi.nlm.nih.gov/pubmed/23271442?tool=bestpractice.com
[57]Million M, Thuny F, Bardin N, et al. Antiphospholipid antibody syndrome with valvular vegetations in acute Q fever. Clin Infect Dis. 2016 Mar 1;62(5):537-44.
http://www.ncbi.nlm.nih.gov/pubmed/26585519?tool=bestpractice.com
[58]Lee CH, Chuah SK, Pei SN, et al. Acute Q fever presenting as antiphospholipid syndrome, pneumonia, and acalculous cholecystitis and masquerading as Mycoplasma pneumoniae and hepatitis C viral infections. Jpn J Infect Dis. 2011;64(6):525-7.
http://www.ncbi.nlm.nih.gov/pubmed/22116335?tool=bestpractice.com
One case of neonatal sepsis due to C burnetii has been reported.[69]Aarthi P, Bagyalakshmi R, Mohan R, et al. First case series of emerging Rickettsial neonatal sepsis identified by polymerase chain reaction-based deoxyribonucleic acid sequencing. Indian J Med Microbiol. 2013 Oct-Dec;31(4):343-8.
http://www.ijmm.org/article.asp?issn=0255-0857;year=2013;volume=31;issue=4;spage=343;epage=348;aulast=Aarthi
http://www.ncbi.nlm.nih.gov/pubmed/24064639?tool=bestpractice.com
Laboratory investigations
Routine tests should include:
Full blood count (FBC): white blood cell (WBC) count may be elevated in approximately 30% of patients. Mild thrombocytopenia (25% of cases) and anaemia may be observed.[5]Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88.
http://www.ncbi.nlm.nih.gov/pubmed/16503466?tool=bestpractice.com
Liver function tests (LFTs): serum transaminases and alkaline phosphatase may be elevated to >2 to 3 times the normal reference range in both acute and persistent focalised infection; bilirubin is normal, but several severe cases of hepatitis or jaundice have been reported.[2]Hartzell JD, Wood-Morris RN, Martinez LJ, et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 May;83(5):574-9.
https://www.mayoclinicproceedings.org/article/S0025-6196(11)60733-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/18452690?tool=bestpractice.com
[70]Suárez Ortega S, Rivero Vera J, Hemmersbach M, et al. Severe cholestatic hepatitis due to Q fever: report of a case [in Spanish]. Gastroenterol Hepatol. 2010 Jan;33(1):21-4.
http://www.ncbi.nlm.nih.gov/pubmed/19819043?tool=bestpractice.com
C-reactive protein (CRP): may be elevated, particularly in cases of persistent focalised infections.
Prolonged activated partial thromboplastin time (aPTT) corresponding to a lupus anticoagulant with anti-prothrombinase activity.
Auto-antibodies: immunoglobulin G (IgG) anticardiolipin (aCL) antibodies are frequently elevated. IgM aCL antibodies and anti-smooth muscle antibodies may also be elevated. IgG aCL antibodies are associated with acute endocarditis, presence of a valvulopathy, progression to endocarditis, and thrombosis when ≥75 G antiphospholipid units (GPLU).[55]Ordi-Ros J, Selva-O'Callaghan A, Monegal-Ferran F, et al. Prevalence, significance, and specificity of antibodies to phospholipids in Q fever. Clin Infect Dis. 1994 Feb;18(2):213-8.
http://www.ncbi.nlm.nih.gov/pubmed/8161629?tool=bestpractice.com
[71]Million M, Walter G, Bardin N, et al. Immunoglobulin G anticardiolipin antibodies and progression to Q fever endocarditis. Clin Infect Dis. 2013 Jul;57(1):57-64.
https://academic.oup.com/cid/article/57/1/57/279982
http://www.ncbi.nlm.nih.gov/pubmed/23532474?tool=bestpractice.com
Cerebrospinal fluid (CSF) analysis: a lumbar puncture should be performed if meningoencephalitis is suspected. Patients may show elevated WBC count with lymphocyte predominance, elevated protein, and normal glucose.[72]Marrie TJ, Raoult D. Rickettsial infections of the central nervous system. Semin Neurol. 1992 Sep;12(3):213-24.
http://www.ncbi.nlm.nih.gov/pubmed/1455109?tool=bestpractice.com
Confirmatory investigations
Diagnosis relies on serology or PCR. Antibody detection by indirect immunofluorescence assay (IFA) is the most commonly used method because of its high sensitivity and specificity. Enzyme immunoassay (EIA) and complement fixation (CF) tests are also used, but they are less accurate. Paradoxically, antibodies to the phase II organism are high in acute disease, and antibodies to the phase I organism are raised in persistent focalised disease.[5]Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88.
http://www.ncbi.nlm.nih.gov/pubmed/16503466?tool=bestpractice.com
Generally, for diagnosis of acute infection, the following must be detected on indirect IFA: phase II antigen IgM titre of 1:50 or higher, and phase II antigen IgG titre of 1:200 or higher, or seroconversion.[2]Hartzell JD, Wood-Morris RN, Martinez LJ, et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 May;83(5):574-9.
https://www.mayoclinicproceedings.org/article/S0025-6196(11)60733-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/18452690?tool=bestpractice.com
However, in the US, the national case definition calls for phase II antigen IgG titre ≥1:128 and does not use IgM for routine diagnostic testing. For diagnosis of persistent focalised infection, one of the following must be detected: phase I antigen IgG of ≥1:800 and low or absent phase II antigen IgM. However, these criteria have now been questioned following the description of endocarditis with low (i.e., 1:200) serological levels in patients with severe heart valve disease during acute infection.[57]Million M, Thuny F, Bardin N, et al. Antiphospholipid antibody syndrome with valvular vegetations in acute Q fever. Clin Infect Dis. 2016 Mar 1;62(5):537-44.
http://www.ncbi.nlm.nih.gov/pubmed/26585519?tool=bestpractice.com
[73]Edouard S, Million M, Lepidi H, et al. Persistence of DNA in a cured patient and positive culture in cases with low antibody levels bring into question diagnosis of Q fever endocarditis. J Clin Microbiol. 2013 Sep;51(9):3012-7.
https://jcm.asm.org/content/51/9/3012.long
http://www.ncbi.nlm.nih.gov/pubmed/23850956?tool=bestpractice.com
Clinical context should, therefore, be carefully considered when reviewing serology results.
PCR may be used for diagnosis of acute infection before initiation of antibiotic therapy (samples should ideally be taken during the first 2 weeks of illness); however, it is not always readily available. It has the advantage of earlier diagnosis compared to serology. PCR testing can be used on tissue samples. It is highly sensitive on tissue samples such as heart valves that have higher numbers of bacteria.[74]Issartel B, Gauduchon V, Chalabreysse L. Clinically and histologically silent Q fever endocarditis accidentally diagnosed by PCR. Clin Microbiol Infect. 2002 Feb;8(2):113-4.
https://onlinelibrary.wiley.com/doi/full/10.1046/j.1198-743x.2001.00360.x
http://www.ncbi.nlm.nih.gov/pubmed/11952725?tool=bestpractice.com
[75]Frangoulidis D, Rodolakis A, Heiser V. DNA microarray-chip based diagnosis of Q-fever (Coxiella burnetii). Clin Microbiol Infect. 2009 Dec;15 Suppl 2:165-6.
http://www.ncbi.nlm.nih.gov/pubmed/19281457?tool=bestpractice.com
PCR can be performed on blood/serum and can yield diagnosis before seroconversion. A technique in which C burnetii DNA is concentrated by lyophilisation has been shown to dramatically increase the sensitivity of this test.[76]Edouard S, Raoult D. Lyophilization to improve the sensitivity of qPCR for bacterial DNA detection in serum: the Q fever paradigm. J Med Microbiol. 2016 Jun;65(6):462-7.
http://www.ncbi.nlm.nih.gov/pubmed/27008653?tool=bestpractice.com
Both serology and PCR can be used to differentiate a patient with symptomatic primo infection (i.e., acute Q fever) from an asymptomatic patient with past conversion, or a patient with persistent focalised infection.[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Natural history of Coxiella burnetii and serological/PCR results. Ac+: positive for antibodies to C burnetii, Ac++: strongly positive for antibodies to C burnetii, Ac-: negative for antibodies to C burnetii, PCR+: positive PCR for C burnetii, PCR++: strongly positive PCR for C burnetii, PCR-: negative PCR for C burnetiiEldin C, et al. Clin Microbiol Rev 2017; used with permission [Citation ends].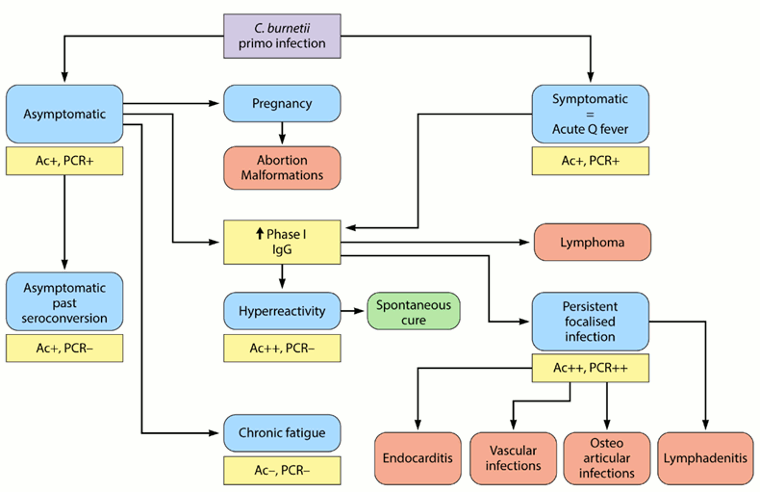
As seroconversion can be delayed up to 6 weeks, serology must be repeated at day 15, 30, and 45 if clinical presentation is consistent but initial serology is negative. PCR on blood/serum, the sensitivity of which can be increased by lyophilisation, can be very useful in this context.[76]Edouard S, Raoult D. Lyophilization to improve the sensitivity of qPCR for bacterial DNA detection in serum: the Q fever paradigm. J Med Microbiol. 2016 Jun;65(6):462-7.
http://www.ncbi.nlm.nih.gov/pubmed/27008653?tool=bestpractice.com
Tissue biopsies, immunohistochemistry, and fluorescence in situ hybridisation (FISH) are the gold standards, but they are performed only in specialist laboratories. Immunohistochemistry has the advantage of being able to identify cell types, while FISH is much more sensitive than immunohistochemistry. Lymph node biopsy should be routinely done in patients with chronic lymphadenitis (as diagnosed by 18F-fluorodeoxyglucose [FDG] positron emission tomography/computed tomography [PET/CT]) due to risk of lymphoma.[64]Melenotte C, Million M, Audoly G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood. 2016 Jan 7;127(1):113-21.
http://www.ncbi.nlm.nih.gov/pubmed/26463422?tool=bestpractice.com
Imaging
Chest x-ray (CXR):
This may be required in acute disease if there is suspicion of pulmonary complications. Findings may range from normal to multiple non-specific (without apparent pattern or distribution) opacities of both lungs most consistent with an atypical pneumonia.[38]Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999 Oct;12(4):518-53.
https://cmr.asm.org/content/12/4/518.full
http://www.ncbi.nlm.nih.gov/pubmed/10515901?tool=bestpractice.com
The most common abnormalities on chest radiography are segmental or lobar opacities. Several rounded opacities are the hallmark of Q fever pneumonia, but they are uncommon. Pleural effusions are rare. In patients with persistent focalised infection, CXR may identify two different persistent focalised infections: interstitial fibrosis and lung pseudotumour.
Echocardiography:
Transthoracic echocardiography (TTE) is routinely recommended during acute infection to exclude underlying cardiac lesions that may be silent, and that might require antibiotic prophylaxis.[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
In patients with acute endocarditis and very high levels of IgG anticardiolipin, a large transient vegetation can often be found.[57]Million M, Thuny F, Bardin N, et al. Antiphospholipid antibody syndrome with valvular vegetations in acute Q fever. Clin Infect Dis. 2016 Mar 1;62(5):537-44.
http://www.ncbi.nlm.nih.gov/pubmed/26585519?tool=bestpractice.com
In patients with chronic endocarditis, vegetations are small or absent.[77]Million M, Raoult D. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013 Jun 13;368(24):2335.
http://www.ncbi.nlm.nih.gov/pubmed/23758255?tool=bestpractice.com
Nodular lesions and calcifications are also frequent.
Transoesophageal echocardiography (TOE) is recommended to identify cardiac lesions in patients aged >40 years who have acute infection with negative TTE and IgG aCL antibodies ≥75 GPLU.[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
Liver ultrasound:
Chest and brain CT scan:
Abdominal CT scan or ultrasound:
These are recommended in patients aged >65 years with acute infection and who currently smoke or have smoked in the past, or have a familial history of aneurysm. Acute infection in patients with aortic aneurysm requires a specific management approach.[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
18F-FDG PET/CT imaging:
Critical for identifying persistent focalised infections.[78]Eldin C, Melenotte C, Million M, et al. 18F-FDG PET/CT as a central tool in the shift from chronic Q fever to Coxiella burnetii persistent focalized infection: a consecutive case series. Medicine (Baltimore). 2016 Aug;95(34):e4287.
http://www.ncbi.nlm.nih.gov/pubmed/27559944?tool=bestpractice.com
This imaging is able to decipher endocarditis, vascular infection, lymphadenitis, and osteoarticular infection, all of which cannot be identified without this technique.
It is now part of the standard anatomical check-up in patients with persistent symptoms, and/or persistent elevated serology, and/or positive PCR on blood/serum, or any sample with clinical presentation not consistent with primary infection. It is specifically recommended for patients with acute infection who have:[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
Persistent phase I IgG ≥1:800 and/or signs of bad clinical evolution
A history of vascular graft or aneurysm
Unexplained (phase I IgG ≥1:800) serology or clinical suspicion of a persistent infection.
It is also useful for identifying infection in patients with vascular prosthesis and/or aneurysm, and identifying those who require surgery with resection of infected vascular tissues.
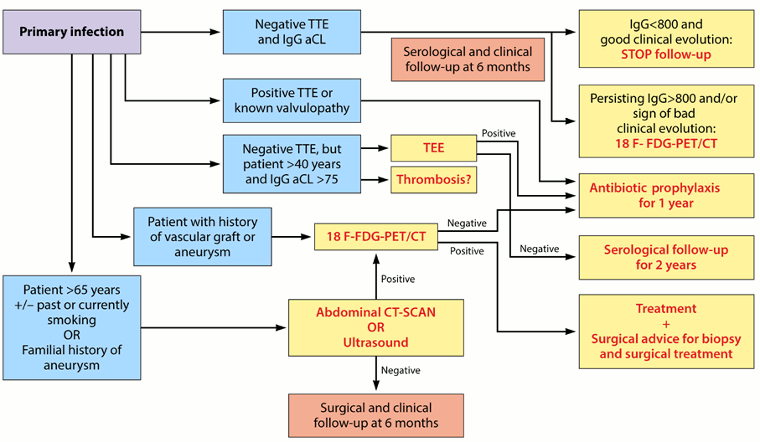
The following algorithm provides a step-by-step approach for managing acute Q fever:[3]Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90.
https://cmr.asm.org/content/30/1/115.long
http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Algorithm for the diagnosis and management of C burnetiiinfection. TTE: transthoracic echocardiography; IgG aCL: IgG anticardiolipin antibodies; 18 F-FDG PET/CT: 18F-fluorodeoxyglucose PET combined with CT Eldin C, et al. Clin Microbiol Rev 2017; used with permission [Citation ends].