Approach
Diagnosis of chronic rhinosinusitis with nasal polyps (CRSwNP) is based on symptoms and endoscopic findings, as well as sinus imaging (computed tomography [CT] scanning), if needed.[2]
At initial presentation, all patients found to have polyps should be examined by an otorhinolaryngologist. A unilateral polyp needs to be differentiated from a nasal tumour and may require biopsy.[25][26]
Nasal polyps in children are rare and, when diagnosed, should prompt referral for testing for cystic fibrosis.[2]
History
The most common symptoms of CRSwNP are:[2]
Nasal obstruction: occurs in 92% of patients
Hyposmia (reduced sense of smell): occurs in 84% of patients
Discoloured nasal discharge or post-nasal drip: occurs in 80% of patients
Facial congestion/pressure/fullness: occurs in 67% of patients
Other symptoms may include headache, halitosis, dental pain, fatigue, cough, dysphonia, sore throat, and sleep difficulties. Bloodstained nasal discharge should arouse suspicion for a nasal tumour.[26]
By definition, chronic rhinosinusitis (CRS) requires symptoms to have lasted for more than 12 weeks.[2]
The European guideline for the diagnosis of CRS defines CRS in adults as the presence of two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip):[2]
with or without facial pain/pressure
with or without reduction or loss of smell
for at least ≥12 weeks, with validation by telephone or interview.
An assessment of the severity and impact of symptoms should be made by asking the patient to mark on a visual analogue scale (VAS) how troublesome their symptoms are, ranging from 0 cm for not troublesome at all to 10 cm for the worst imaginable level. The disease may then be categorised as mild, moderate, or severe based on the result:[2]
Mild = VAS score 0-3
Moderate = VAS score >3 to 7
Severe = VAS score >7 to 10
CRS is broadly divided into CRSwNP and CRS sine/without nasal polyps (CRSsNP). Differentiation of CRSwNP from CRSsNP requires endoscopic or CT identification of nasal polyps.
Direct visualisation of polyps
A widened bridge of the nose on external examination may be suggestive of polyps, but clinical examination relies mainly on a combination of anterior rhinoscopy and nasal endoscopy.[27] Nasal endoscopy can be performed with a flexible or rigid endoscope, typically after a topical decongestant and local anaesthetic have been applied to the nasal mucosa. These examinations confirm inflammation, which is necessary for the diagnosis of chronic rhinosinusitis, and at the same time allow direct visualisation of polyps. However, small polyps may evade detection. Large polyps can be seen by anterior rhinoscopy, or simply by using the largest attachment of an otoscope, and are distinguishable from the inferior turbinate by their lack of sensitivity, grey colour, and absence of a connection with the lateral wall of the nose.[27][Figure caption and citation for the preceding image starts]: Grade 3 nasal polypsFrom the collection of Dr Richard Hewitt [Citation ends].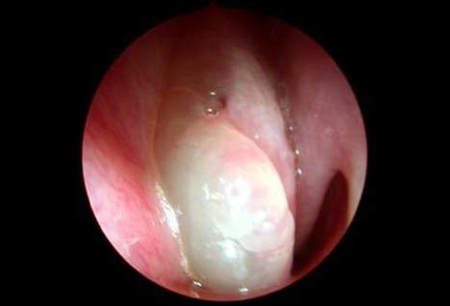
Video outlining how to perform an examination of the nose and nasal cavity
The endoscopic appearance of polyps is graded as follows:[28]
Grade 0: absence of polyps
Grade 1: small polyps in the middle meatus not reaching below the inferior border of the middle turbinate
Grade 2: polyps reaching below the inferior border of the middle turbinate
Grade 3: large polyps reaching the lower border of the inferior turbinate or nasal polyps medial to the middle turbinate
Grade 4: polyps completely obstructing the nasal cavity
Imaging
CT scanning is the modality of choice for confirmation of the extent of the disease and assessment of the underlying anatomy of the nose and paranasal sinuses.[2] Nasal polyps appear as soft-tissue opacifications of the sinuses and nasal cavity.
CT scanning is an important investigation in the diagnostic work-up of the condition, particularly if there are features that are concerning for neoplasia, such as unilateral symptoms, epistaxis/bleeding, crusting, cacosmia (perception of foul odour), periorbital oedema, displaced globe, double or reduced vision, ophthalmoplegia, severe frontal headaches, frontal swelling, signs of meningitis or focal neurology. CT scanning is also required prior to endoscopic sinus surgery.[2]
Plain x-rays are not indicated because they are insensitive and of limited use in the evaluation of nasal polyps and the sinuses.[2]
Other investigations
CRSwNP can usually be diagnosed based on history plus endoscopic examination and/or sinus CT scan. Additional investigations may sometimes be indicated.
Biopsy
Indicated if there are concerns about alternative diagnoses, particularly neoplasia.[2]
Nasal smear and culture
A nasal smear demonstrating the presence of eosinophils is typical in CRSwNP, but may also be seen in allergic rhinitis and non-allergic rhinitis with eosinophilia syndrome. Nasal swabs/nasal mucus culture may be beneficial in revealing secondary bacterial infection, although Staphylococcus aureus carriage in the nose is very common.[2][27]
FBC with differential
Patients with CRSwNP often have a peripheral blood eosinophil count at the upper end of the normal range or modestly elevated. Levels of 0.4 × 10⁶ cells/L up to 1.5 × 10⁶ cells/L may be seen, with higher levels in patients with asthma. Levels above 1.0 × 10⁶ cells/L also raise the possibility of eosinophilic granulomatous polyangiitis (EGPA, also known as Churg-Strauss syndrome) if accompanied by additional features such as palpable purpura and fleeting pulmonary infiltrates.[29]
Allergy testing
Skin prick tests and serum allergen-specific IgE tests are useful in the investigation of patients presenting with rhinitis symptoms where they can help confirm or exclude a diagnosis of allergic rhinitis. Skin test results themselves do not contribute to the diagnosis of CRSwNP, which occurs in both atopic and non-atopic individuals.[2]
Aspirin provocation
All patients with chronic rhinosinusitis should be asked if they have experienced reactions to aspirin/non-steroidal anti-inflammatory drugs (NSAIDs) previously. Aspirin provocation/challenge is recommended when the history is unclear.[2]
Aspirin provocation/challenge can be performed by nasal, inhaled, or oral routes. It is most commonly done as an oral challenge, but nasal challenge with lysine aspirin is a safer alternative.[30] It must be carried out only by clinicians with appropriate experience and with full resuscitation facilities readily available.
Approximately 5% to 30% of patients with nasal polyps have concomitant aspirin/NSAID hypersensitivity.[31] Patients found to have aspirin sensitivity should be warned to avoid all drugs with COX-1 inhibitory activity. Selective COX-2 inhibitors appear to be safe in patients with aspirin sensitivity, although it is recommended that the first dose is administered in hospital under direct observation, with monitoring for 2 hours after completion.
A positive challenge informs the need to avoid aspirin and NSAIDs in future; aspirin desensitisation may also be considered as a treatment measure in appropriate patients.[2]
Erythrocyte sedimentation rate (ESR) and anti-neutrophil cytoplasmic antibody (ANCA)
Indicated when EGPA is suspected, for example in patients with severe asthma and CRSwNP, blood eosinophilia and additional constitutional symptoms, vasculitic rash or neurological symptoms/signs. Thirty-five percent of patients with EGPA are positive for ANCA.[2] ESR is frequently raised. Referral to a specialist is advised if this condition is suspected.
Olfaction studies
Tests such as the University of Pennsylvania Smell Identification Test are well validated and can be used to assess the impact of CRSwNP on olfaction.[32]
Quality-of-life measures
Questionnaire-based assessments such as the Sinonasal Outcome Test (SNOT-20 or SNOT-22) allow assessment of severity and impact of the condition.[2]
Nasal airway assessment
Peak nasal inspiratory flow is quick and easy to use. It is used in some specialist rhinology clinics. Flow rates are not standardised and calibrated to age, height, and sex in the same way that peak expiratory flow rates are, but repeat measures can be useful, particularly before and after treatment.
Use of this content is subject to our disclaimer
