Investigations
1st investigations to order
Blastomyces dermatitidis enzyme immunoassay (EIA) urine antigen testing
Test
EIA urine antigen testing is indicated in the first instance, with reported sensitivity varying from 76% to 90%.[32]
Although urine samples are preferred, the assay is also clinically available for use on serum, cerebrospinal fluid (CSF) or bronchoalveolar lavage samples.[2][32] Sensitivity ranges from 80% to 93% depending on the sample and clinical manifestation of disease, although sensitivity may be as low as 55% in routine clinical settings.[2][40] False positive rates of 2% in the healthy population and less than 5% in other invasive fungal infections lead to a higher specificity than serology.[3]
False-positive results due to cross-reactivity can occur in patients with Histoplasma infection, however cross-reactivity is unlikely to change therapy.[30][31][32]
Result
positive for B dermatitidis galactomannan antigen
chest x-ray
Test
Posteroanterior and lateral chest x-ray should be obtained in all patients suspected of having blastomycosis to document pulmonary involvement and to guide invasive diagnostic strategies.[2] However, there are no imaging findings that are specific enough to blastomycosis to establish the diagnosis. [Figure caption and citation for the preceding image starts]: Pulmonary blastomycosis with focal infiltrates on chest x-rayDr Robert Orenstein, DO, Associate Professor of Medicine, Division of Infectious Diseases, Mayo Clinic, Scottsdale, AZ [Citation ends].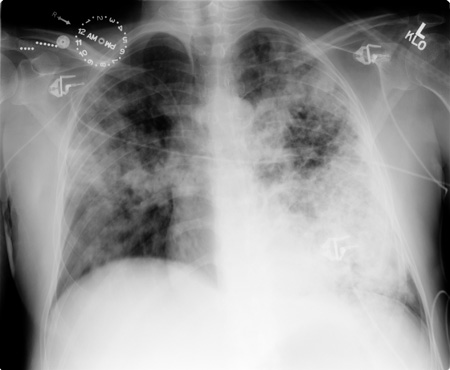 [Figure caption and citation for the preceding image starts]: Pulmonary blastomycosis presenting as a miliary pattern on chest x-rayDr Robert Orenstein, DO, Associate Professor of Medicine, Division of Infectious Diseases, Mayo Clinic, Scottsdale, AZ [Citation ends].
[Figure caption and citation for the preceding image starts]: Pulmonary blastomycosis presenting as a miliary pattern on chest x-rayDr Robert Orenstein, DO, Associate Professor of Medicine, Division of Infectious Diseases, Mayo Clinic, Scottsdale, AZ [Citation ends].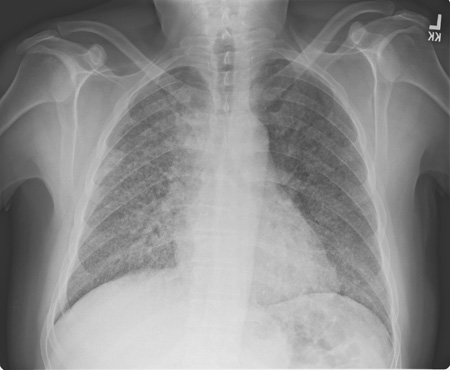 [Figure caption and citation for the preceding image starts]: Close-up of reticulonodular findings on chest x-ray in disseminated blastomycosisPersonal files of Larry Baddour, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Close-up of reticulonodular findings on chest x-ray in disseminated blastomycosisPersonal files of Larry Baddour, MD [Citation ends].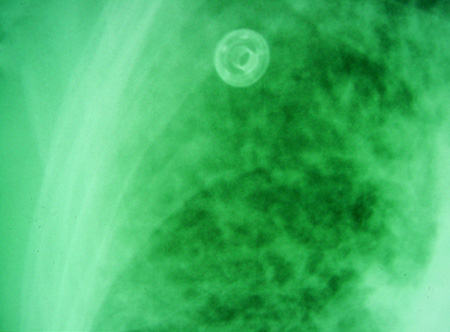
Result
lobar pneumonia, mass-like or cavitary lesions, diffuse interstitial infiltrates, or signs of adult respiratory distress syndrome
respiratory tract specimen smear and culture
Test
Confirmatory of the diagnosis and can be performed with potassium hydroxide preparations or special fungal stains.[2][35][Figure caption and citation for the preceding image starts]: Direct potassium hydroxide preparation at 40x magnification of a sputum specimenNancy L. Wengenack, PhD, D(ABMM), Director of Mycology and Mycobacteriology Laboratories, Assistant Professor of Microbiology abd Lab Medicine, Mayo Clinic, Rochester, MN [Citation ends]. [Figure caption and citation for the preceding image starts]: Direct potassium hydroxide preparation at 20x magnification of a sputum specimenNancy L. Wengenack, PhD, D(ABMM), Director of Mycology and Mycobacteriology Laboratories, Assistant Professor of Microbiology and Lab Medicine, Mayo Clinic, Rochester, MN [Citation ends].
[Figure caption and citation for the preceding image starts]: Direct potassium hydroxide preparation at 20x magnification of a sputum specimenNancy L. Wengenack, PhD, D(ABMM), Director of Mycology and Mycobacteriology Laboratories, Assistant Professor of Microbiology and Lab Medicine, Mayo Clinic, Rochester, MN [Citation ends].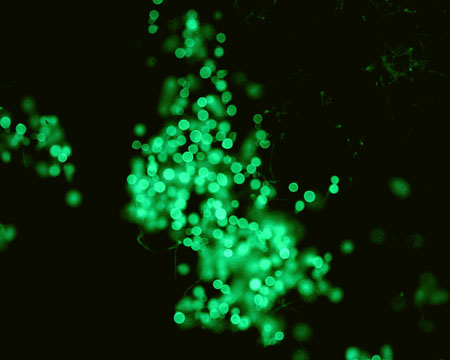
From expectorated specimen or bronchoalveolar lavage.
Demonstration of typical yeast in fluid or tissue specimens is usually sufficient to start treatment for blastomycosis. However, definitive diagnosis usually requires culture confirmation, which can take 2 to 4 weeks.
Culture on Sabouraud dextrose agar or potato dextrose agar with and without cycloheximide should be incubated at 25 to 30°C for up to 6 weeks.[2] The off-white mould form should be tested for Blastomyces dermatitidis with a commercially available DNA probe assay.
Result
large, oval, broad-based budding yeast; or positive DNA probe assay result
Investigations to consider
bronchoscopy
Test
Performed when sputum samples are unobtainable or non-revealing in patients with pulmonary involvement.
Result
washings with large, oval, broad-based budding yeast; or tissue specimen with granuloma
tissue biopsy or cytology (lung, skin, or bone)
Test
Transbronchial biopsy of lung lesions, or biopsy of suspicious skin or bone lesions.
Result
acute inflammation with or without necrosis, granuloma formation, and multi-nucleated giant cells
arthrocentesis
Test
A negative Gram stain and culture may be a clue to the disease.
Result
large, oval, broad-based budding yeast in joint fluid
fungal serology panel
Test
Including complement fixation, immunodiffusion, and enzyme immunoassay, this measures antibodies to Blastomyces dermatitidis.
Frequently ordered, but notoriously low sensitivity (9% to 64%) and specificity (50% to 67%) and generally not useful in making the diagnosis.[2][3]
Useful only if positive.
Repeat testing is not recommended regardless of the initial test result.
Result
may be positive
fungal blood cultures
Test
Positive fungal blood cultures can be seen in disseminated disease and offer a non-invasive diagnostic strategy. A negative fungal blood culture does not rule out disseminated disease.
Can take up to 2 weeks for results.
Result
positive
MRI brain
Test
Central nervous system involvement occurs in 5% to 10% of cases of blastomycosis and can manifest as meningitis or as a space-occupying lesion.[23]
MRI may reveal evidence of intracranial blastomycosis. [Figure caption and citation for the preceding image starts]: Axial T1 gadolinium-enhanced MRI image of cerebral blastomycosisDr William Marshall, MD, Assistant Professor of Medicine, Division of Infectious Diseases, Mayo Clinic, Rochester, MN [Citation ends].
Result
positive
B dermatitidis polymerase chain reaction
Use of this content is subject to our disclaimer