Hidradenitis suppurativa
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
acute abscess
antibiotic therapy
Guidelines recommend a 12-week course of a tetracycline antibiotic.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com
In severe HS presenting with a disease flare, rescue therapy in the form of a 6-week course of intravenous ertapenem, followed by a 6-week course of consolidation treatment with moxifloxacin plus metronidazole plus rifampicin, may be considered.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [45]Chahine AA, Nahhas AF, Braunberger TL, et al. Ertapenem rescue therapy in hidradenitis suppurativa. JAAD Case Rep. 2018 Jun;4(5):482-3. http://www.ncbi.nlm.nih.gov/pubmed/29984290?tool=bestpractice.com [46]Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016 Feb;71(2):513-20. https://www.doi.org/10.1093/jac/dkv361 http://www.ncbi.nlm.nih.gov/pubmed/26565016?tool=bestpractice.com
Primary options
tetracycline: 500 mg orally twice daily
OR
doxycycline: 100 mg orally twice daily
OR
minocycline: 100 mg orally twice daily
Secondary options
ertapenem: 1 g intravenously every 24 hours
and
moxifloxacin: 400 mg orally once daily
and
metronidazole: 500 mg orally three times daily
and
rifampicin: 300 mg orally twice daily
intralesional corticosteroid
Treatment recommended for ALL patients in selected patient group
If the patient is well, intralesional corticosteroids may provide relief from pain and inflammation, in combination with oral antibiotics if required.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [47]Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016 Dec;75(6):1151-5. http://www.ncbi.nlm.nih.gov/pubmed/27692735?tool=bestpractice.com
Primary options
triamcinolone acetonide: consult specialist for guidance on dose
incision and drainage
Additional treatment recommended for SOME patients in selected patient group
If the patient is unwell or disease does not improve with antibiotics and/or intralesional corticosteroids, incision and drainage may be considered.[44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com Incision and drainage is a supplemental measure; it should not be considered as the sole treatment because recurrence is very common.
mild (Hurley stage I)
topical antibacterial or antibiotic
Stage I is defined as the presence of localised disease with inflammatory papules, pustules, nodules, and abscesses but without sinus tracts or scarring.[4]Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus. In: Roenigk RH, Roenigk HH Jr, eds. Dermatologic surgery: principles and practice. New York, NY: Marcel Dekker; 1989:729-739.[Figure caption and citation for the preceding image starts]: Hidradenitis suppurativa stage I: discrete inflamed nodules and papules with intervening normal skin and lack of scarringFrom R.A. Lee, MD, PhD [Citation ends].
It is recommended that patients use an antimicrobial wash, but there is no strong evidence for specific agents; use of chlorhexidine, benzoyl peroxide, and zinc pyrithione is supported by expert opinion. Concomitant use of an antimicrobial wash may be associated with lower rates of antibiotic resistance in HS lesions.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [48]Leiphart P, Ma H, Naik HB, et al. The effect of antimicrobial washes on antibacterial resistance in hidradenitis suppurativa lesions. J Am Acad Dermatol. 2019 Mar;80(3):821-2. https://www.doi.org/10.1016/j.jaad.2018.10.063 http://www.ncbi.nlm.nih.gov/pubmed/30403961?tool=bestpractice.com
Topical antibiotics are useful treatments in mild HS.[41]Martorell A, García FJ, Jiménez-Gallo D, et al. Update on hidradenitis suppurativa (part II): treatment. Actas Dermosifiliogr. 2015;106:716-724. http://www.actasdermo.org/en/update-on-hidradenitis-suppurative-part/articulo/S1578219015002449 http://www.ncbi.nlm.nih.gov/pubmed/26277040?tool=bestpractice.com Topical clindamycin has been shown to be effective in a clinical trial setting.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [49]Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-4. http://www.ncbi.nlm.nih.gov/pubmed/9843011?tool=bestpractice.com Topical metronidazole is another option. Topical therapy should be continued for a minimum of 8 weeks before evaluation of efficacy.
Primary options
clindamycin topical: (1%) apply to the affected area(s) twice daily
OR
metronidazole topical: (0.75%) apply to the affected area(s) twice daily; (1%) apply to the affected area(s) once daily
antibiotic therapy
Additional treatment recommended for SOME patients in selected patient group
Oral tetracyclines are recommended first-line for mild disease. They can attenuate neutrophil activity and reduce pain and inflammation. A treatment course of 12-weeks is recommended before evaluation of efficacy.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com
Primary options
tetracycline: 500 mg orally twice daily
OR
doxycycline: 100 mg orally twice daily
OR
minocycline: 100 mg orally twice daily
analgesia
Additional treatment recommended for SOME patients in selected patient group
The degree of pain usually correlates with the degree of inflammation. Thus, treatments directed at inflammation are often effective at alleviating pain. Non-steroidal anti-inflammatory drugs should be used as required before other pain medications such as paracetamol.
Primary options
ibuprofen: 400 mg orally every 4-6 hours when required, maximum 2400 mg/day
Secondary options
paracetamol: 500-1000 mg orally every 4-6 hours when required, maximum 4000 mg/day
lifestyle modifications
Additional treatment recommended for SOME patients in selected patient group
A high proportion of patients with hidradentitis suppurativa are active smokers or have a history of smoking, and are obese. Obesity is an independent risk factor for development of the disease and contributes to HS disease severity.[21]Tzellos T, Zouboulis CC, Gulliver W, et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015 Nov;173(5):1142-55. http://www.ncbi.nlm.nih.gov/pubmed/26153913?tool=bestpractice.com [24]Jørgensen AR, Yao Y, Ghazanfar MN, et al. Burden, predictors and temporal relationships of comorbidities in patients with hidradenitis suppurativa: a hospital-based cohort study. J Eur Acad Dermatol Venereol. http://www.ncbi.nlm.nih.gov/pubmed/31442338?tool=bestpractice.com All patients should be advised to stop smoking, to lose weight if obese, and to be evaluated for cardiovascular disease.[44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [50]Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014 Sep;94(5):553-7. https://www.doi.org/10.2340/00015555-1800 http://www.ncbi.nlm.nih.gov/pubmed/24577555?tool=bestpractice.com
moderate (Hurley stage II)
antibiotic therapy
Stage II is defined as the presence of inflammatory papules and nodules, ≥1 recurring, widely separated abscesses with sinus tracts and scarring.[4]Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus. In: Roenigk RH, Roenigk HH Jr, eds. Dermatologic surgery: principles and practice. New York, NY: Marcel Dekker; 1989:729-739.[41]Martorell A, García FJ, Jiménez-Gallo D, et al. Update on hidradenitis suppurativa (part II): treatment. Actas Dermosifiliogr. 2015;106:716-724.
http://www.actasdermo.org/en/update-on-hidradenitis-suppurative-part/articulo/S1578219015002449
http://www.ncbi.nlm.nih.gov/pubmed/26277040?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Hidradenitis suppurativa stage II: inflamed nodules and scars with areas of intervening normal skinFrom R.A. Lee, MD, PhD [Citation ends].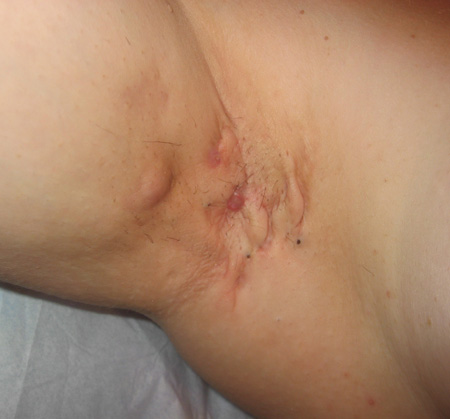
Tetracyclines are recommended for 12 weeks.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com
Clindamycin plus rifampicin is an effective combination and should be continued for 10 to 12 weeks.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com However, patients often relapse within 4 to 5 months of stopping therapy.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [51]Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-54. http://www.ncbi.nlm.nih.gov/pubmed/19590173?tool=bestpractice.com
Diarrhoea may reduce tolerability of clindamycin plus rifampicin in some patients.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com Patients who are aged ≥50 years or ever-smokers are less likely to tolerate this combination.[36]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019 Jul;81(1):76-90. https://www.doi.org/10.1016/j.jaad.2019.02.067 http://www.ncbi.nlm.nih.gov/pubmed/30872156?tool=bestpractice.com [52]Schneller-Pavelescu L, Vergara-de Caso E, Martorell A, et al. Interruption of oral clindamycin plus rifampicin therapy in patients with hidradenitis suppurativa: An observational study to assess prevalence and causes. J Am Acad Dermatol. 2019 May;80(5):1455-7. https://www.doi.org/10.1016/j.jaad.2018.12.043 http://www.ncbi.nlm.nih.gov/pubmed/30630028?tool=bestpractice.com [53]Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015 Apr;29(4):619-44. http://www.ncbi.nlm.nih.gov/pubmed/25640693?tool=bestpractice.com
Rifampicin induces the cytochrome P450 system; check for potential drug interactions with existing medication including the oral contraceptive pill. Clindamycin plus rifampicin may also select for rifampicin-resistant strains of Mycobacterium tuberculosis; tuberculosis screening or avoiding this regimen may be indicated in high-risk populations.[54]Mendes-Bastos P, Macedo R, Duarte R. Treatment of hidradenitis suppurativa with rifampicin: have we forgotten tuberculosis? Br J Dermatol. 2017 Oct;177(4):e150-e151. http://www.ncbi.nlm.nih.gov/pubmed/28718933?tool=bestpractice.com
Triple antibiotic therapy with moxifloxacin plus metronidazole plus rifampicin is a second-line option for moderate HS.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com
Primary options
tetracycline: 500 mg orally twice daily
OR
doxycycline: 100 mg orally twice daily
OR
minocycline: 100 mg orally twice daily
OR
clindamycin: 300 mg orally twice daily
and
rifampicin: 300 mg orally twice daily
Secondary options
moxifloxacin: 400 mg orally once daily
and
metronidazole: 500 mg orally three times daily
and
rifampicin: 300 mg orally twice daily
topical antibiotic or antibacterial
Treatment recommended for ALL patients in selected patient group
It is recommended that patients use an antimicrobial wash, but there is no strong evidence for specific agents; use of chlorhexidine, benzoyl peroxide, and zinc pyrithione is supported by expert opinion. Concomitant use of an antimicrobial wash may be associated with lower rates of antibiotic resistance in HS lesions.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [48]Leiphart P, Ma H, Naik HB, et al. The effect of antimicrobial washes on antibacterial resistance in hidradenitis suppurativa lesions. J Am Acad Dermatol. 2019 Mar;80(3):821-2. https://www.doi.org/10.1016/j.jaad.2018.10.063 http://www.ncbi.nlm.nih.gov/pubmed/30403961?tool=bestpractice.com These products are available over the counter.
Topical antibiotics are useful treatments in mild HS.[41]Martorell A, García FJ, Jiménez-Gallo D, et al. Update on hidradenitis suppurativa (part II): treatment. Actas Dermosifiliogr. 2015;106:716-724. http://www.actasdermo.org/en/update-on-hidradenitis-suppurative-part/articulo/S1578219015002449 http://www.ncbi.nlm.nih.gov/pubmed/26277040?tool=bestpractice.com Topical clindamycin has been shown to be effective in a clinical trial setting.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [49]Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-4. http://www.ncbi.nlm.nih.gov/pubmed/9843011?tool=bestpractice.com Topical metronidazole is another option. Topical therapy should be continued for a minimum of 8 weeks before evaluation of efficacy.
Primary options
clindamycin topical: (1%) apply to the affected area(s) twice daily
OR
metronidazole topical: (0.75%) apply to the affected area(s) twice daily; (1%) apply to the affected area(s) once daily
analgesia
Additional treatment recommended for SOME patients in selected patient group
The degree of pain usually correlates with the degree of inflammation. Thus, treatments directed at inflammation are often effective at alleviating pain. Non-steroidal anti-inflammatory drugs should be used as required before other pain medications such as paracetamol.
Primary options
ibuprofen: 400 mg orally every 4-6 hours when required, maximum 2400 mg/day
Secondary options
paracetamol: 500-1000 mg orally every 4-6 hours when required, maximum 4000 mg/day
lifestyle modifications
Additional treatment recommended for SOME patients in selected patient group
A high proportion of patients with hidradentitis suppurativa are active smokers or have a history of smoking, and are obese. Obesity is an independent risk factor for development of the disease and contributes to HS disease severity.[21]Tzellos T, Zouboulis CC, Gulliver W, et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015 Nov;173(5):1142-55. http://www.ncbi.nlm.nih.gov/pubmed/26153913?tool=bestpractice.com [24]Jørgensen AR, Yao Y, Ghazanfar MN, et al. Burden, predictors and temporal relationships of comorbidities in patients with hidradenitis suppurativa: a hospital-based cohort study. J Eur Acad Dermatol Venereol. http://www.ncbi.nlm.nih.gov/pubmed/31442338?tool=bestpractice.com All patients should be advised to stop smoking, to lose weight if obese, and to be evaluated for cardiovascular disease.[44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [50]Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014 Sep;94(5):553-7. https://www.doi.org/10.2340/00015555-1800 http://www.ncbi.nlm.nih.gov/pubmed/24577555?tool=bestpractice.com
dapsone
Dapsone is reserved for patients with moderate disease who have failed multiple courses of combination antibiotics.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com Evidence is limited; in one published case series, 25% patients experienced clinically significant improvement but with rapid disease recurrence upon cessation.[55]Yazdanyar S, Boer J, Ingvarsson G, et al. Dapsone therapy for hidradenitis suppurativa: a series of 24 patients. Dermatology. 2011;222(4):342-6. http://www.ncbi.nlm.nih.gov/pubmed/21757878?tool=bestpractice.com
Primary options
dapsone: 50-200 mg orally once daily
topical antibiotic or antibacterial
Treatment recommended for ALL patients in selected patient group
It is recommended that patients use an antimicrobial wash, but there is no strong evidence for specific agents; use of chlorhexidine, benzoyl peroxide, and zinc pyrithione is supported by expert opinion. Concomitant use of an antimicrobial wash may be associated with lower rates of antibiotic resistance in HS lesions.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [48]Leiphart P, Ma H, Naik HB, et al. The effect of antimicrobial washes on antibacterial resistance in hidradenitis suppurativa lesions. J Am Acad Dermatol. 2019 Mar;80(3):821-2. https://www.doi.org/10.1016/j.jaad.2018.10.063 http://www.ncbi.nlm.nih.gov/pubmed/30403961?tool=bestpractice.com These products are available over the counter.
Topical antibiotics are useful treatments in mild HS.[41]Martorell A, García FJ, Jiménez-Gallo D, et al. Update on hidradenitis suppurativa (part II): treatment. Actas Dermosifiliogr. 2015;106:716-724. http://www.actasdermo.org/en/update-on-hidradenitis-suppurative-part/articulo/S1578219015002449 http://www.ncbi.nlm.nih.gov/pubmed/26277040?tool=bestpractice.com Topical clindamycin has been shown to be effective in a clinical trial setting.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [49]Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-4. http://www.ncbi.nlm.nih.gov/pubmed/9843011?tool=bestpractice.com Topical metronidazole is another option. Topical therapy should be continued for a minimum of 8 weeks before evaluation of efficacy.
Primary options
clindamycin topical: (1%) apply to the affected area(s) twice daily
OR
metronidazole topical: (0.75%) apply to the affected area(s) twice daily; (1%) apply to the affected area(s) once daily
analgesia
Additional treatment recommended for SOME patients in selected patient group
The degree of pain usually correlates with the degree of inflammation. Thus, treatments directed at inflammation are often effective at alleviating pain. Non-steroidal anti-inflammatory drugs should be used as required before other pain medications such as paracetamol.
Primary options
ibuprofen: 400 mg orally every 4-6 hours when required, maximum 2400 mg/day
Secondary options
paracetamol: 500-1000 mg orally every 4-6 hours when required, maximum 4000 mg/day
lifestyle modifications
Additional treatment recommended for SOME patients in selected patient group
A high proportion of patients with hidradentitis suppurativa are active smokers or have a history of smoking, and are obese. Obesity is an independent risk factor for development of the disease and contributes to HS disease severity.[21]Tzellos T, Zouboulis CC, Gulliver W, et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015 Nov;173(5):1142-55. http://www.ncbi.nlm.nih.gov/pubmed/26153913?tool=bestpractice.com [24]Jørgensen AR, Yao Y, Ghazanfar MN, et al. Burden, predictors and temporal relationships of comorbidities in patients with hidradenitis suppurativa: a hospital-based cohort study. J Eur Acad Dermatol Venereol. http://www.ncbi.nlm.nih.gov/pubmed/31442338?tool=bestpractice.com All patients should be advised to stop smoking, to lose weight if obese, and to be evaluated for cardiovascular disease.[44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [50]Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014 Sep;94(5):553-7. https://www.doi.org/10.2340/00015555-1800 http://www.ncbi.nlm.nih.gov/pubmed/24577555?tool=bestpractice.com
spironolactone
Additional treatment recommended for SOME patients in selected patient group
North American guidelines suggest that hormonal agents, including spironolactone, may be considered in women with clear premenstrual flares (while recognising that recommendations regarding hormonal therapies are based on limited evidence).[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com
Use of spironolactone should be limited to women who are practising adequate birth control.
Treatment should be continued for at least 8 weeks.
UK guidelines conclude that there is insufficient evidence to recommend anti-androgens for the treatment of HS.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [56]Nikolakis G, Kyrgidis A, Zouboulis CC. Is there a role for antiandrogen therapy for hidradenitis suppurativa? A systematic review of published data. Am J Clin Dermatol. 2019 Aug;20(4):503-13. http://www.ncbi.nlm.nih.gov/pubmed/31073704?tool=bestpractice.com
Primary options
spironolactone: 100-150 mg orally once daily
oral retinoid
Additional treatment recommended for SOME patients in selected patient group
Patients with concomitant acne vulgaris may consider using oral isotretinoin.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [57]Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-6. http://www.ncbi.nlm.nih.gov/pubmed/9922015?tool=bestpractice.com
Before treatment, patients require counselling about the potential adverse effects. Severe headaches, decreased night vision, or signs of adverse psychiatric events are indications for prompt discontinuation. Full blood count, lipid panel, and liver function tests are monitored regularly.
Isotretinoin is teratogenic; therefore, women undergo pregnancy testing before starting isotretinoin and monthly while taking the drug. In the UK, isotretinoin is prescribed under the Pregnancy Prevention Programme (PPP), while in the US, it can only be prescribed through the iPledge system.[65]Medicines and Healthcare products Regulatory Agency. Oral retinoids: pregnancy prevention - reminder of measures to minimise teratogenic risk. December 2014 [internet publication]. https://www.gov.uk/drug-safety-update/oral-retinoids-pregnancy-prevention-reminder-of-measures-to-minimise-teratogenic-risk iPledge system (for isotretinoin prescribing) Opens in new window These programmes are aimed at decreasing the number of birth defects associated with this medicine. Oral isotretinoin should be continued for at least 6 months.
Acitretin has demonstrated moderate efficacy in HS.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [58]Boer J, Nazary M. Long-term results of acitretin therapy for hidradenitis suppurativa. Br J Dermatol. 2010;164:170-5. http://www.ncbi.nlm.nih.gov/pubmed/20874789?tool=bestpractice.com Treatment duration in excess of 6 months has been reported.[59]Blok JL, van Hattem S, Jonkman MF, et al. Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. Br J Dermatol. 2013;168:243-52. http://www.ncbi.nlm.nih.gov/pubmed/23106519?tool=bestpractice.com Acitretin is teratogenic and should be avoided in women of child-bearing potential.
Primary options
isotretinoin: 0.5 to 1 mg/kg/day orally given in 2 divided doses
Secondary options
acitretin: 25-50 mg orally once daily
surgical repair
Additional treatment recommended for SOME patients in selected patient group
Wide excision with wide and deep margins is the standard of care for surgical therapy. Because of the size of the excision and its corresponding repair, and the availability of specific lasers, referral to plastic surgery and/or a dermatological surgeon is advisable.[60]van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. http://www.ncbi.nlm.nih.gov/pubmed/20708472?tool=bestpractice.com [61]Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. http://www.ncbi.nlm.nih.gov/pubmed/19438670?tool=bestpractice.com [62]Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. http://www.ncbi.nlm.nih.gov/pubmed/20227579?tool=bestpractice.com [63]Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. http://www.ncbi.nlm.nih.gov/pubmed/20039918?tool=bestpractice.com
Local excision is possible for smaller, quiescent lesions where the clinical margins can be clearly defined.
Destructive methods, such as cryotherapy, are generally not recommended. Selective use of deroofing of individual epithelialised sinus tracts can be effective in treating specific recurrent lesions.
severe (Hurley stage III)
antibiotic therapy
Stage III disease is defined as multiple abscesses and interconnected sinus tracts and scars.[4]Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus. In: Roenigk RH, Roenigk HH Jr, eds. Dermatologic surgery: principles and practice. New York, NY: Marcel Dekker; 1989:729-739.[Figure caption and citation for the preceding image starts]: Hidradenitis suppurativa stage III: interconnected scars, cysts, comedones, and inflamed nodulesFrom R.A. Lee, MD, PhD [Citation ends].
In practice, patients in this group will have previously used oral tetracyclines, and they may be used again in between other measures to maintain disease control in stage III disease. However, once disease reaches stage III more aggressive treatment is usually required.
In selected stage III patients with severe HS presenting with a disease flare (usually after failure of a tetracycline [for 12 weeks] or clindamycin plus rifampicin), a 6-week course of intravenous ertapenem, followed by a 6-week course of consolidation treatment with moxifloxacin plus metronidazole plus rifampicin, may be considered.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [45]Chahine AA, Nahhas AF, Braunberger TL, et al. Ertapenem rescue therapy in hidradenitis suppurativa. JAAD Case Rep. 2018 Jun;4(5):482-3. http://www.ncbi.nlm.nih.gov/pubmed/29984290?tool=bestpractice.com [46]Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016 Feb;71(2):513-20. https://www.doi.org/10.1093/jac/dkv361 http://www.ncbi.nlm.nih.gov/pubmed/26565016?tool=bestpractice.com
Primary options
tetracycline: 500 mg orally twice daily
OR
doxycycline: 100 mg orally twice daily
OR
minocycline: 100 mg orally twice daily
OR
clindamycin: 300 mg orally twice daily
and
rifampicin: 300 mg orally twice daily
Secondary options
ertapenem: 1 g intravenously every 24 hours
and
moxifloxacin: 400 mg orally once daily
and
metronidazole: 500 mg orally three times daily
and
rifampicin: 300 mg orally twice daily
topical antibiotic or antibacterial
Treatment recommended for ALL patients in selected patient group
It is recommended that all patients use an antimicrobial wash, but there is no strong evidence for specific agents; use of chlorhexidine, benzoyl peroxide, and zinc pyrithione is supported by expert opinion. Concomitant use of an antimicrobial wash may be associated with lower rates of antibiotic resistance in HS lesions.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [48]Leiphart P, Ma H, Naik HB, et al. The effect of antimicrobial washes on antibacterial resistance in hidradenitis suppurativa lesions. J Am Acad Dermatol. 2019 Mar;80(3):821-2. https://www.doi.org/10.1016/j.jaad.2018.10.063 http://www.ncbi.nlm.nih.gov/pubmed/30403961?tool=bestpractice.com These products are available over the counter.
Topical antibiotics are useful treatments in mild HS.[41]Martorell A, García FJ, Jiménez-Gallo D, et al. Update on hidradenitis suppurativa (part II): treatment. Actas Dermosifiliogr. 2015;106:716-724. http://www.actasdermo.org/en/update-on-hidradenitis-suppurative-part/articulo/S1578219015002449 http://www.ncbi.nlm.nih.gov/pubmed/26277040?tool=bestpractice.com Topical clindamycin has been shown to be effective in a clinical trial setting.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [49]Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-4. http://www.ncbi.nlm.nih.gov/pubmed/9843011?tool=bestpractice.com Topical metronidazole is another option. Topical therapy should be continued for a minimum of 8 weeks before evaluation of efficacy.
Primary options
clindamycin topical: (1%) apply to the affected area(s) twice daily
OR
metronidazole topical: (0.75%) apply to the affected area(s) twice daily; (1%) apply to the affected area(s) once daily
analgesia
Additional treatment recommended for SOME patients in selected patient group
The degree of pain usually correlates with the degree of inflammation. Thus, treatments directed at inflammation are often effective at alleviating pain. Non-steroidal anti-inflammatory drugs should be used as required before other pain medications such as paracetamol.
Primary options
ibuprofen: 400 mg orally every 4-6 hours when required, maximum 2400 mg/day
Secondary options
paracetamol: 500-1000 mg orally every 4-6 hours when required, maximum 4000 mg/day
tumour necrosis factor (TNF)-alpha inhibitor
Additional treatment recommended for SOME patients in selected patient group
Adalimumab has demonstrated efficacy in phase 3 trials of HS.[64]Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-34. http://www.nejm.org/doi/full/10.1056/NEJMoa1504370#t=article http://www.ncbi.nlm.nih.gov/pubmed/27518661?tool=bestpractice.com It is the only approved biologic for HS, and is therefore considered the first-choice biological agent in moderate/severe HS refractory to conventional treatments.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com
Infliximab may be considered as a second-line biological agent, and is used off-label for HS.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com
Other biological agents may be considered if adalimumab or infliximab fail or are contraindicated, but their use is off-label and should be under specialist guidance.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com
Therapy with biologics is continued for at least 12 weeks and the efficacy of treatment assessed at this time.
Primary options
adalimumab: 160 mg subcutaneously on day 1, followed by 80 mg on day 15, then 40 mg once weekly or 80 mg every 2 weeks starting on day 29
Secondary options
infliximab: consult specialist for guidance on dose
lifestyle modifications
Additional treatment recommended for SOME patients in selected patient group
A high proportion of patients with hidradentitis suppurativa are active smokers or have a history of smoking, and are obese. Obesity is an independent risk factor for development of the disease and contributes to HS disease severity.[21]Tzellos T, Zouboulis CC, Gulliver W, et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015 Nov;173(5):1142-55. http://www.ncbi.nlm.nih.gov/pubmed/26153913?tool=bestpractice.com [24]Jørgensen AR, Yao Y, Ghazanfar MN, et al. Burden, predictors and temporal relationships of comorbidities in patients with hidradenitis suppurativa: a hospital-based cohort study. J Eur Acad Dermatol Venereol. http://www.ncbi.nlm.nih.gov/pubmed/31442338?tool=bestpractice.com All patients should be advised to stop smoking, to lose weight if obese, and to be evaluated for cardiovascular disease.[44]Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):19-31. https://www.doi.org/10.1111/jdv.15233 http://www.ncbi.nlm.nih.gov/pubmed/30176066?tool=bestpractice.com [50]Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014 Sep;94(5):553-7. https://www.doi.org/10.2340/00015555-1800 http://www.ncbi.nlm.nih.gov/pubmed/24577555?tool=bestpractice.com
spironolactone
Additional treatment recommended for SOME patients in selected patient group
North American guidelines suggest that hormonal agents, including spironolactone, may be considered in women with clear premenstrual flares (while recognising that recommendations regarding hormonal therapies are based on limited evidence).[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com
Use of spironolactone should be limited to women who are practising adequate birth control.
Treatment should be continued for at least 8 weeks.
UK guidelines conclude that there is insufficient evidence to recommend anti-androgens for the treatment of HS.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [56]Nikolakis G, Kyrgidis A, Zouboulis CC. Is there a role for antiandrogen therapy for hidradenitis suppurativa? A systematic review of published data. Am J Clin Dermatol. 2019 Aug;20(4):503-13. http://www.ncbi.nlm.nih.gov/pubmed/31073704?tool=bestpractice.com
Primary options
spironolactone: 100-150 mg orally once daily
oral retinoid
Additional treatment recommended for SOME patients in selected patient group
Patients with concomitant acne vulgaris may consider using oral isotretinoin.[42]Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019 May;180(5):1009-17. https://www.doi.org/10.1111/bjd.17537 http://www.ncbi.nlm.nih.gov/pubmed/30552762?tool=bestpractice.com [43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [57]Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-6. http://www.ncbi.nlm.nih.gov/pubmed/9922015?tool=bestpractice.com
Before treatment, patients require counselling about the potential adverse effects. Severe headaches, decreased night vision, or signs of adverse psychiatric events are indications for prompt discontinuation. Full blood count, lipid panel, and liver function tests are monitored regularly.
Isotretinoin is teratogenic; therefore, women undergo pregnancy testing before starting isotretinoin and monthly while taking the drug. In the UK, isotretinoin is prescribed under the Pregnancy Prevention Programme (PPP), while in the US, it can only be prescribed through the iPledge system.[65]Medicines and Healthcare products Regulatory Agency. Oral retinoids: pregnancy prevention - reminder of measures to minimise teratogenic risk. December 2014 [internet publication]. https://www.gov.uk/drug-safety-update/oral-retinoids-pregnancy-prevention-reminder-of-measures-to-minimise-teratogenic-risk iPledge system (for isotretinoin prescribing) Opens in new window These programmes are aimed at decreasing the number of birth defects associated with this medicine. Oral isotretinoin should be continued for at least 6 months.
Acitretin has demonstrated moderate efficacy in HS.[43]Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101. https://www.doi.org/10.1016/j.jaad.2019.02.068 http://www.ncbi.nlm.nih.gov/pubmed/30872149?tool=bestpractice.com [58]Boer J, Nazary M. Long-term results of acitretin therapy for hidradenitis suppurativa. Br J Dermatol. 2010;164:170-5. http://www.ncbi.nlm.nih.gov/pubmed/20874789?tool=bestpractice.com Treatment duration in excess of 6 months has been reported.[59]Blok JL, van Hattem S, Jonkman MF, et al. Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. Br J Dermatol. 2013;168:243-52. http://www.ncbi.nlm.nih.gov/pubmed/23106519?tool=bestpractice.com Acitretin is teratogenic and should be avoided in women of child-bearing potential.
Primary options
isotretinoin: 0.5 to 1 mg/kg/day orally given in 2 divided doses
Secondary options
acitretin: 25-50 mg orally once daily
surgical repair
Additional treatment recommended for SOME patients in selected patient group
Wide excision with wide and deep margins is the standard of care for surgical therapy. Because of the size of the excision and its corresponding repair, and the availability of specific lasers, referral to plastic surgery and/or a dermatological surgeon is advisable.[60]van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. http://www.ncbi.nlm.nih.gov/pubmed/20708472?tool=bestpractice.com [61]Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. http://www.ncbi.nlm.nih.gov/pubmed/19438670?tool=bestpractice.com [62]Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. http://www.ncbi.nlm.nih.gov/pubmed/20227579?tool=bestpractice.com [63]Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. http://www.ncbi.nlm.nih.gov/pubmed/20039918?tool=bestpractice.com
Local excision is possible for smaller, quiescent lesions where the clinical margins can be clearly defined.
Destructive methods, such as cryotherapy, are generally not recommended. Selective use of deroofing of individual epithelialised sinus tracts can be effective in treating specific recurrent lesions.

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer