White ethnicity, female sex, prematurity, low birth weight, multiple gestation, and advanced maternal age are strong risk factors for development of haemangioma.
History and physical examination
Infantile haemangiomas may be present at birth, but they more typically present during the first few weeks of life as flat pink or blue macules or patches.[2]Garzon MC. Infantile hemangioma. In: Callen JP, Horn TD, Mancini AJ, et al, eds. Dermatology. Vol. 2. 2nd ed. St. Louis, MO: Elsevier; 2008:1565-80.[16]Bruckner AL, Friedan IJ. Hemangiomas of infancy. J Am Acad Dermatol. 2003 Apr;48(4):477-93.
http://www.ncbi.nlm.nih.gov/pubmed/12664009?tool=bestpractice.com
Alternatively, they may present as a red or blue papule or nodule. They undergo a period of accelerated growth, known as the 'proliferative phase', helping to differentiate them from other vascular entities. The duration of the proliferative phase varies depending on the morphology of the infantile haemangioma, but 80% of growth is usually reached by 3 months of age.[14]Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015 Oct;136(4):e1060-104.
http://pediatrics.aappublications.org/content/136/4/e1060
http://www.ncbi.nlm.nih.gov/pubmed/26416931?tool=bestpractice.com
A superficial haemangioma develops a bright red colour: the surface appears tight and tense. Ulceration and bleeding may occur, particularly in areas subject to increased friction, maceration, and trauma (e.g., nappy area, neck, axilla, and scalp). Most lesions reach a period of stability by age 6 to 12 months, and then enter a period of spontaneous involution. Involution may take several years, during which the red colour changes to blue, grey, and/or pink. Islands of normal-coloured skin become apparent, and the haemangioma is palpably softer.[2]Garzon MC. Infantile hemangioma. In: Callen JP, Horn TD, Mancini AJ, et al, eds. Dermatology. Vol. 2. 2nd ed. St. Louis, MO: Elsevier; 2008:1565-80.[6]Haggstrom AN, Lammer EJ, Schneider RA, et al. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics. 2006 Mar;117(3):698-703.
http://www.ncbi.nlm.nih.gov/pubmed/16510649?tool=bestpractice.com
[23]Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006 Sep;118(3):882-7.
http://www.ncbi.nlm.nih.gov/pubmed/16950977?tool=bestpractice.com
Deep haemangiomas feel tense, and may swell with crying and dependent positioning. They lack the characteristic red or pink colour, and may appear later in the first year of life. Their growth phase may not be as apparent and may occur later than superficial infantile haemangiomas, raising the possibility of confusion with a vascular malformation. Midline facial lesions, particularly those over the nose, need to be confidently differentiated from dermoid cysts, gliomas, and encephaloceles. Haemangioma overlying the lumbosacral spine may mimic meningocele and myelomeningocele.[10]Grevelink SV, Mulliken JB. Vascular anomalies and tumors of skin and subcutaneous tissues. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick's dermatology in general medicine. Vol. 1. 6th ed. New York, NY: McGraw-Hill; 2003:1002-26.
Imaging studies
Imaging studies are typically unnecessary but may differentiate infantile haemangioma from vascular or lymphatic malformations when diagnosis is uncertain, or for deep lesions which can be difficult to assess physically.[24]American College of Radiology. ACR appropriateness criteria: soft tissue vascular anomalies: vascular malformations and infantile vascular tumors (non-CNS)-child. 2023 [internet publication].
https://acsearch.acr.org/docs/3186695/Narrative
Doppler ultrasound of the lesion is quick, accurate, and cost effective. Ultrasound avoids the risks of anaesthesia. However, interpretation is highly dependent upon the expertise of the technician; and it may not conclusively distinguish proliferative vascular tumours (e.g., infantile haemangioma) from vascular malformations.
Magnetic resonance imaging (MRI) is indicated when clinical examination and ultrasound fail to provide a diagnosis, or when the extent of the lesion and relationship to adjacent structures needs to be defined more fully.[24]American College of Radiology. ACR appropriateness criteria: soft tissue vascular anomalies: vascular malformations and infantile vascular tumors (non-CNS)-child. 2023 [internet publication].
https://acsearch.acr.org/docs/3186695/Narrative
[25]Paltiel HJ, Burrows PE, Kozakewich HP, et al. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000 Mar;214(3):747-54.
http://www.ncbi.nlm.nih.gov/pubmed/10715041?tool=bestpractice.com
MRI with contrast may differentiate infantile haemangioma from venous, arteriovenous, and lymphatic malformations.[26]Kern S, Niemeyer C, Darge K, et al. Differentiation of vascular birthmarks by MR imaging. An investigation of hemangiomas, venous and lymphatic malformations. Acta Radiol. 2000 Sep;41(5):453-7.
http://www.ncbi.nlm.nih.gov/pubmed/11016765?tool=bestpractice.com
MRI can also be used to distinguish an infantile haemangioma from an encephalocele or meningocele/myelomeningocele.[10]Grevelink SV, Mulliken JB. Vascular anomalies and tumors of skin and subcutaneous tissues. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick's dermatology in general medicine. Vol. 1. 6th ed. New York, NY: McGraw-Hill; 2003:1002-26. MRI studies are a useful adjunct to distinguish infantile haemangioma from malignant soft-tissue masses, including fibrosarcoma. Diagnostic specificity is reported to be up to 90%, with negative predictive value for malignancy up to 94%.[27]Kitami M. Diffusion-weighted imaging as a routine MRI protocol for the evaluation of 'infantile hemangioma'. Clin Imaging. 2017 Nov - Dec;46:121.
http://www.ncbi.nlm.nih.gov/pubmed/28780146?tool=bestpractice.com
By contrast, the specificity for benign vascular tumours is 50%, and the specificity for malignant vascular tumours is 80%. A tissue biopsy is indicated when malignancy cannot be excluded.[1]Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019 Jan;143(1): e20183475.
https://pediatrics.aappublications.org/content/143/1/e20183475.long
http://www.ncbi.nlm.nih.gov/pubmed/30584062?tool=bestpractice.com
Computed tomography (CT) is not as useful as MRI because a CT scan cannot identify patterns of vascular flow.[10]Grevelink SV, Mulliken JB. Vascular anomalies and tumors of skin and subcutaneous tissues. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick's dermatology in general medicine. Vol. 1. 6th ed. New York, NY: McGraw-Hill; 2003:1002-26. Arteriography has been replaced in modern practice largely by non-invasive imaging techniques.[10]Grevelink SV, Mulliken JB. Vascular anomalies and tumors of skin and subcutaneous tissues. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick's dermatology in general medicine. Vol. 1. 6th ed. New York, NY: McGraw-Hill; 2003:1002-26.
MRI is also the test of choice to evaluate associated anomalies of the spine, brain, etc., when considering syndromic infantile haemangioma.[14]Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015 Oct;136(4):e1060-104.
http://pediatrics.aappublications.org/content/136/4/e1060
http://www.ncbi.nlm.nih.gov/pubmed/26416931?tool=bestpractice.com
Biopsy histology
Histopathological findings vary greatly depending on the phase at which the infantile haemangioma is biopsied. Proliferative haemangiomas have a lobular architecture and are highly cellular. They involve the dermis and may extend into the subcutaneous tissues. Subtle vascular lumina appear slit-like and are surrounded by plump endothelial cells. Normal-appearing mitoses are frequent. Mast cells populate the surrounding stroma.[14]Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015 Oct;136(4):e1060-104.
http://pediatrics.aappublications.org/content/136/4/e1060
http://www.ncbi.nlm.nih.gov/pubmed/26416931?tool=bestpractice.com
[28]Weedon D. Vascular tumors. In: Houston MJ, ed. Skin pathology. 2nd ed. New York, NY: Churchill Livingston; 2002:1001-43. With involution, lumina enlarge and endothelial cells flatten. Vessels are replaced by fibro-fatty tissue.[28]Weedon D. Vascular tumors. In: Houston MJ, ed. Skin pathology. 2nd ed. New York, NY: Churchill Livingston; 2002:1001-43. Haemangiomas uniquely stain for GLUT1, an erythrocyte-type glucose transporter present in infantile haemangioma, brain, and placenta.[29]North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangioma. Hum Pathol. 2000 Jan;31(1):11-22.
http://www.ncbi.nlm.nih.gov/pubmed/10665907?tool=bestpractice.com
[30]Bree AF, Siegfried E, Sotelo-Avila C, et al. Infantile hemangioma: speculation on placental trophoblastic origin. Arch Dermatol. 2001 May;137(5):573-7.
http://jamanetwork.com/journals/jamadermatology/fullarticle/478335
http://www.ncbi.nlm.nih.gov/pubmed/11346335?tool=bestpractice.com
[31]North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathological entity unlike infantile hemangioma. Arch Dermatol. 2001 Dec;137(12):1607-20.
http://jamanetwork.com/journals/jamadermatology/fullarticle/478613
http://www.ncbi.nlm.nih.gov/pubmed/11735711?tool=bestpractice.com
If a malignant tumour with a significant vascular component is considered a possibility, biopsy with histopathological examination and special tissue stains, including GLUT1, is indicated.[28]Weedon D. Vascular tumors. In: Houston MJ, ed. Skin pathology. 2nd ed. New York, NY: Churchill Livingston; 2002:1001-43.
Variants and special considerations
Segmental haemangiomas may be associated with underlying abnormalities. Segmental cervicofacial, upper chest, shoulder, or arm haemangiomas may be associated with structural anomalies of the brain, cerebral vasculature, eyes, sternum, and/or aorta. This neurocutaneous disorder is known as PHACE(S) syndrome, with the acronym referring to posterior fossa anomalies, haemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, abnormalities of the eye, and sternal clefting or supraumbilical raphe.[8]Metry D, Heyer G, Hess C, et al. Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics. 2009 Nov;124(5):1447-56.
http://www.ncbi.nlm.nih.gov/pubmed/19858157?tool=bestpractice.com
The syndrome is often incomplete.[32]Metry DW, Dowd CF, Barkovich AJ, et al. The many faces of PHACE syndrome. J Pediatr. 2001 Jul;139(1):117-23. [Erratum in: J Pediatr 2001 Sep;139(3):470.]
http://www.ncbi.nlm.nih.gov/pubmed/11445804?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Tender, ulcerated haemangioma on the left lower lipFrom the collection of Carla T. Lane, MD, PhD; used with permission [Citation ends].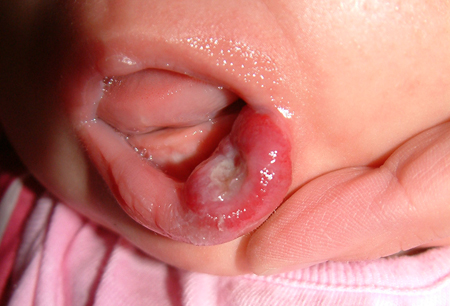 Infants with segmental cervicofacial haemangioma require ophthalmological examination, echocardiogram, and possible central nervous system imaging.
Infants with segmental cervicofacial haemangioma require ophthalmological examination, echocardiogram, and possible central nervous system imaging.
Beard haemangioma: haemangiomas located on the lower face and neck have been associated with laryngeal haemangioma.[1]Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019 Jan;143(1): e20183475.
https://pediatrics.aappublications.org/content/143/1/e20183475.long
http://www.ncbi.nlm.nih.gov/pubmed/30584062?tool=bestpractice.com
Progressive stridor is a worrisome sign. CT with intravenous (IV) contrast may be useful when optimal imaging of the airway is required.[24]American College of Radiology. ACR appropriateness criteria: soft tissue vascular anomalies: vascular malformations and infantile vascular tumors (non-CNS)-child. 2023 [internet publication].
https://acsearch.acr.org/docs/3186695/Narrative
Infants with haemangiomas in a beard distribution should be referred to an otolaryngologist for further evaluation and possible endoscopy.[1]Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019 Jan;143(1): e20183475.
https://pediatrics.aappublications.org/content/143/1/e20183475.long
http://www.ncbi.nlm.nih.gov/pubmed/30584062?tool=bestpractice.com
Lumbosacral haemangioma: haemangioma located in the lumbosacral area may signal underlying spinal dysraphism. Other associated malformations include tethered cord, renal, and skeletal anomalies. MRI is the test of choice.[9]Goldberg NS, Hebert AA, Esterly NB. Sacral hemangioma and multiple congenital abnormalities. Arch Dermatol. 1986 Jun;122(6):684-7.
http://www.ncbi.nlm.nih.gov/pubmed/3717979?tool=bestpractice.com
[10]Grevelink SV, Mulliken JB. Vascular anomalies and tumors of skin and subcutaneous tissues. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick's dermatology in general medicine. Vol. 1. 6th ed. New York, NY: McGraw-Hill; 2003:1002-26.[24]American College of Radiology. ACR appropriateness criteria: soft tissue vascular anomalies: vascular malformations and infantile vascular tumors (non-CNS)-child. 2023 [internet publication].
https://acsearch.acr.org/docs/3186695/Narrative
Segmental perineal, genital, buttock, or thigh haemangiomas should raise concern for LUMBAR syndrome, which refers to lower body infantile haemangioma and other cutaneous defects, urogenital anomalies and ulceration, myelopathy, bony deformities, anorectal malformations and arterial anomalies, and renal anomalies.[1]Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019 Jan;143(1): e20183475.
https://pediatrics.aappublications.org/content/143/1/e20183475.long
http://www.ncbi.nlm.nih.gov/pubmed/30584062?tool=bestpractice.com
Multifocal cutaneous infantile haemangiomas: infants with multiple cutaneous haemangiomas may have haemangiomas within their visceral organs. A prospective study revealed that 16% of infants who present with ≥5 infantile haemangiomas have hepatic haemangiomas.[12]Horii KA, Drolet BA, Frieden IJ, et al. Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2011 May-Jun;28(3):245-53.
http://www.ncbi.nlm.nih.gov/pubmed/21517952?tool=bestpractice.com
In such patients a good physical examination is indicated. Hepatomegaly may indicate clinically significant liver haemangiomas and should be evaluated by ultrasound.[24]American College of Radiology. ACR appropriateness criteria: soft tissue vascular anomalies: vascular malformations and infantile vascular tumors (non-CNS)-child. 2023 [internet publication].
https://acsearch.acr.org/docs/3186695/Narrative
An abnormal cardiac examination may indicate high-output heart failure. Patients with multifocal cutaneous infantile haemangiomas or large visceral lesions are also at risk for hypothyroidism.[14]Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015 Oct;136(4):e1060-104.
http://pediatrics.aappublications.org/content/136/4/e1060
http://www.ncbi.nlm.nih.gov/pubmed/26416931?tool=bestpractice.com
Haemangiomas in certain locations can result in significant cosmetic or functional complications. Periorbital haemangiomas may result in ocular complications. Haemangiomas on the nasal tip or ear may cause cartilage destruction. Lesions on the face and ears may lead to permanent disfigurement. Bulky lesions on the scalp may result in alopecia. Lip haemangiomas can cause feeding problems and distort the normal contour of the mouth.[13]Paller A, Mancini A. Hurwitz clinical pediatric dermatology. 4th ed. Philadelphia, PA: Saunders; 2011:268-302. Genital and perineal haemangiomas are more likely to ulcerate and lead to associated complications. [Figure caption and citation for the preceding image starts]: Plaque-type cervicofacial ulcerated haemangioma (beard distribution)From the collection of Carla T. Lane, MD, PhD; used with permission [Citation ends]. Breast haemangiomas in females may result in permanent changes in breast development or nipple contour.
Breast haemangiomas in females may result in permanent changes in breast development or nipple contour.
 Infants with segmental cervicofacial haemangioma require ophthalmological examination, echocardiogram, and possible central nervous system imaging.
Infants with segmental cervicofacial haemangioma require ophthalmological examination, echocardiogram, and possible central nervous system imaging.  Breast haemangiomas in females may result in permanent changes in breast development or nipple contour.
Breast haemangiomas in females may result in permanent changes in breast development or nipple contour.