Aetiology
The term anabolic-androgenic steroid (AAS) covers drugs designed to increase androgenic action by direct administration of testosterone or its derivatives, or by using drugs that increase endogenous testosterone production, such as by increasing luteinising hormone (LH) that then acts on Leydig cells to produce testosterone.[1]
Drug classes of AASs based on their mechanism of action:[1][6]
Exogenous androgens (includes endogenous androgens used as drugs)
Testosterone
Dihydrotestosterone (DHT)
Nandrolone
Synthetic testosterone derivatives (e.g., danazol, stanozolol)
Androgen precursors
Androstenedione
Androsterone
Dehydroepiandrosterone (DHEA)
Gonadotrophins:
Human chorionic gonadotrophin
Recombinant human LH
Aromatase inhibitors
Anastrozole
Letrozole
Exemestane
Selective oestrogen receptor modulators
Clomiphene
Raloxifene
Most AASs are obtained over the internet (over 50%).[9] The majority of internet sources of AASs are for 'designer steroids' produced in research or underground laboratories.[10]
Most users of AASs seek improved muscle appearance, strength, or performance, while some are trying to improve self-esteem or wellbeing.[6][11][12] One survey of the motives for AAS use reported responses for the non-anabolic effects of AASs, including to prepare for criminal activity.[13] However, aggression and criminality is more likely to be an AAS-induced association in some individuals.[14]
AAS use correlates with both illicit drug use and prescription drug abuse.[15][16][17]
Pathophysiology
Anabolic-androgenic steroids (AASs) comprise synthetic testosterone derivatives.[18] Testosterone is thought to be metabolised by 5-alpha-reductase to dihydrotestosterone (DHT). DHT acts in the cell nucleus of target tissues and is primarily responsible for the androgenic (i.e., masculinising) and anabolic (i.e., tissue-building) effects. Testosterone is also metabolised by aromatase to estradiol.[19] Under normal physiological conditions, aromatase plays a limited role. High-dose AAS increases the amounts of oestrogens produced, which may lead to irreversible feminising effects in men.
Taking testosterone results in negative feedback inhibition of the hypothalamic-pituitary-testicular axis and suppression of gonadotrophin-releasing hormone (GnRH), luteinising hormone (LH), follicle-stimulating hormone (FSH), and testosterone production.[19] Testicular atrophy and decreased sperm count and mobility result. Infertility can occur within months. Other adverse effects due to AAS use in men include acne, oily skin, muscular development, changes in libido, erectile dysfunction, temporal hairline recession, and gynaecomastia. In women, adverse effects include acne, oily skin, muscular development, breast atrophy, menstrual irregularities, and changes in libido. The potential irreversible masculinising effects in women include hirsutism, male pattern baldness, deepening of the voice, and clitoral hypertrophy.[19][20] Psychiatric effects include impulse control, aggression, anxiety, and hypomania or mania while using high-dose AAS, with anxiety and depression likely once AASs are stopped.[1]
Children and adolescents experience accelerated maturation associated with changes in physique and earlier development of secondary sexual characteristics. Premature closure of growth plates in long bones can occur leading to a decrease in final height.[21]
Hepatic effects are not hormonally related, but are instead due to chemical manipulation of the testosterone structure. Unpredictable and transient elevations in liver function tests are noted in AAS users but are usually of muscular origin due to rhabdomyolysis caused by heavy workouts, rather than liver origin.[22] Cholestasis is a complication of the 17-alpha alkyl-substituted drugs. Most athletes avoid these or discontinue when icterus develops. Peliosis hepatis and hepatic tumours are also a recognised complication of the 17-alpha alkyl-substituted drugs.
AAS use enhances vascular resistance and blood pressure and the pro-inflammatory biomarker profile; alters serum lipoproteins; and produces direct myocardial toxicity.[23] Cardiovascular complications include dyslipidaemia with accelerated atherogenesis, electrolyte and fluid abnormalities (i.e., water and salt retention) with chronic hypertension, cardiomyopathy, left ventricular hypertrophy, myocardial ischaemia, arrhythmias, increased platelet aggregation, erythropoiesis, and thrombotic events.[2][23] These effects are suggested to be the cause of premature death in AAS users.[2] The cause of cardiovascular toxicity is not well understood, but several models have been proposed:[24]
The atherogenic model suggests lipoprotein abnormalities as the cause. Atherogenesis may be due to increased triglyceride lipase activity, which catabolises very low-density lipoprotein (VLDL) to low-density lipoprotein (LDL) and also catabolises high-density lipoprotein (HDL). These changes are reversible.
The thrombotic model suggests hypercoagulability. AAS may have a direct effect on the coagulation/fibrinolytic system to increase coagulation factors.
The vasospastic model cites endothelial dysfunction and nitric oxide changes.
A model of direct cardiovascular toxicity suggests cell injury by disruption of myocardial mitochondria and induction of collagen dysplasia. Cell death occurs, scar tissue replaces healthy tissue, and fibrosis ensues. Hypertension develops and can be followed by left ventricular hypertrophy and structural changes to the ventricular wall.
Loss of autonomic control of the heart rate, particularly impaired parasympathetic slowing of the heart rate at rest and after exercise.[25]
Classification
Anabolic-androgenic steroid classification
Classified according to their pharmacology and chemical structure.
17-beta ester derivatives
Boldenone
Mibolerone
Nandrolone
Trenbolone.
1-methyl derivatives
Mesterolone
Methenolone
Testosterone esters.
17-alpha alkyl derivatives
Danazol
Ethylestrenol
Fluoxymesterone
Methandrostenolone
Methyltestosterone
Oxandrolone
Oxymesterone
Oxymetholone
Stanozolol.
Other
7-alpha-methyl-19-nortestosterone
Tetrahydrogestrinone.
[Figure caption and citation for the preceding image starts]: Structures of several common anabolic-androgenic steroidsUS National Library of Medicine: ChemIDPlus [Citation ends].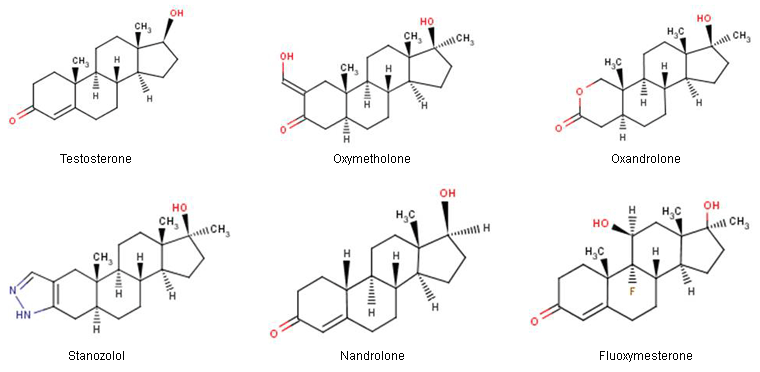
Anabolic-androgenic steroid administration
Can be given orally, parenterally, transdermally, or subcutaneously.
Patterns of administration include cycling, pyramiding, stacking, and bridging.[2]
Cycling
Involves using different combinations of drugs over a period of time, usually 6 to 12 weeks, stopping for a period of time to allow normal hormonal production to return, before starting again. In the 'post-cycle' period, users of AAS will try different drugs and strategies to offset the adverse effects of low endogenous testosterone levels, such as gynaecomastia and hypogonadism (testicular atrophy, infertility, low libido, erectile dysfunction).
Stacking
Taking ≥2 different AASs by different routes, usually with other drugs such as human growth hormone for perceived synergistic effect.
Pyramiding
Starting with low doses and then slowly increasing to a maximum level, and then slowly decreasing again. Usually this is completed over 6 to 12 weeks and is sometimes followed by a drug-free training period. The pyramid decrease is usually completed within 1 to 2 months of competition.
Bridging/blast and cruise
Alternating between periods of high and low doses of AAS, but never completely ceasing drug use.
Use of this content is subject to our disclaimer