Etiology
Wilms tumor is a genetically heterogeneous neoplasm.[3][14] It may be inherited or occur sporadically. The cancer genes that underpin Wilms tumor are diverse and involve around 40 genes.[3] Most genes act in gene expression control and growth factor signaling.[3] Germline mutations are present in at least 10% of patients and include the first gene implicated in Wilms tumorigenesis, the WT1 (Wilms tumor 1) gene.[15] Other germline mutations detected in the Children's Oncology Group TARGET initiative paper include TP53 (tumor protein p53; 17p13), DICER1 (dicer 1, ribonuclease III; 14q32), DIS3L2 (DIS3 like 3'-5' exoribonuclease 2; 2q37), and some unexpected germline variants involving PALB2 (partner and localizer of BRCA2; 16p12) and CHEK2 (checkpoint kinase 2; 22q12).[15] The most prevalent somatic mutations are in the WT1 (11p13), CTNNB1 (catenin beta 1; 3p22), AMER1 (APC membrane recruitment protein 1; Xq11), IGF2 (insulin-like growth factor 2; 11p15), TP53 (17p13), MYCN (MYCN proto-oncogene, bHLH transcription factor; 2p24), SIX1 (SIX homeobox 1; 14q23) and SIX2 (SIX homeobox 2; 2p21), and microRNA processing genes.[14]
The risk for developing Wilms tumor is increased in children with congenital anomalies. In one study of childhood cancer in Great Britain, congenital anomalies were found in around 8% of children with Wilms tumor.[16] The risk is increased among children with certain congenital overgrowth syndromes, such as Perlman syndrome, Beckwith-Wiedemann syndrome, and Simpson-Golabi-Behmel syndrome, and also among children with certain congenital nonovergrowth syndromes, such as Denys-Drash syndrome and WAGR (Wilms tumor, aniridia, genitourinary abnormalities, range of developmental delays) syndrome.[3][17]
Children with congenital urogenital anomalies such as hypospadias, atypical genitalia, fused (horseshoe) kidney, or cryptorchidism are also predisposed to developing Wilms tumor.[3][16][18]
Studies have investigated the association between Wilms tumor and prenatal exposure to harmful environmental factors or maternal lifestyle risk factors; most studies are inconclusive or require further investigation. However, a meta-analysis of case-control studies identified a link between parental pesticide exposure during the preconception or pregnancy period and an increased risk for Wilms tumor.[19]
Pathophysiology
Many different genetic changes converge into a limited number of developmental pathways resulting in Wilms tumor oncogenesis.[15] By understanding these identified pathways, potential targeted therapeutic approaches can be applied in a clinical setting.[3]
Wilms tumor oncogenesis is thought to be a multistep process that arises from aberrant fetal nephrogenesis, with many Wilms tumor-related genes having pivotal roles in the developing kidney.[3][14] As the differentiation arrest of renal progenitor cells is incomplete, all three lineages of the developing kidney (blastema, epithelia, and stroma) can be identified in classic triphasic Wilms tumor histology.[14]
Nephrogenic rests are microscopic foci of primitive blastemal renal elements found in normal kidney tissue in an intralobar or perilobar location, and are derived from renal stem cells. Nephrogenic rests are found in >90% of patients with bilateral Wilms tumors and in around 40% of patients with unilateral sporadic Wilms tumor, and are considered precursor lesions to Wilms tumor.[3] The acquisition of additional genetic and epigenetic changes then leads to tumor formation.
The first genetic model, or "two-hit hypothesis" (two rate-limiting mutational events that occur sequentially), postulates that children who develop hereditary Wilms tumor are born with a constitutional prezygotic DNA mutation in one allele of a gene, which are initiating events, and that this event is followed by another genetic event in the same or different gene locus, leading to formation of the tumor.[20][21] The WT1 gene (11p13) and the WT2 gene (11p15) are tumor suppressor genes that are known to play an important role in the normal development of the urogenital tract.[20] Inactivation of these genes appears to be an early genetic event in the evolution of Wilms tumor. Loss of heterozygosity (LOH) at 1p and 16q is thought to be a secondary event that ultimately results in Wilms tumor formation.[20]
Studies suggest that aberrant expression of the IGF-2 oncogene, resulting from the loss of imprinting of the maternal IGF-2 gene, may be one of the most common epigenetic alterations in Wilms tumor.[22] Furthermore, intralobar nephrogenic rests are more likely to be associated with the identical genetic mutation found in the Wilms tumors originating in the same host.[23]
Altogether, these studies suggest that both nephrogenic rests and the tumor may be clonally derived from a common embryonic renal stem cell and that multiple sequential genetic and epigenetic events such as inactivation of tumor suppressor genes and LOH at 1p and 16q may occur within these lesions leading to tumorigenesis.[24] An analysis of histologically normal tissue also supports the hypothesis that clonal expansions within normal kidney tissue, some of which harbor driver variants, antedate the development of Wilms tumor.[25]
Copy number gains at the chromosome region 1q21.1-q31.3 have been shown to be significantly associated with relapse, as well as allelic imbalances at chromosome arms 1p, 1q, 3p, 3q, and 14q.[26][27]
Analysis of primary-relapse tumor pairs (where DNA is available from the primary tumor to compare with the relapse tumor) suggests that WT2 (11p15) LOH (and other copy number changes) and mutations in WT1 and MLLT1 typically occur early, but mutations in SIX1, MYCN, and WTX are late developments.[28]
[Figure caption and citation for the preceding image starts]: Chromosome 11 deletionNational Cancer Institute; used with permission [Citation ends].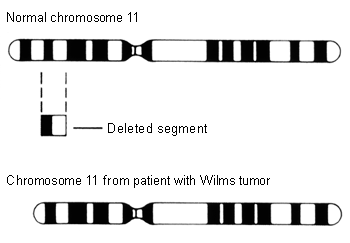
Use of this content is subject to our disclaimer