Ehrlichiosis and anaplasmosis have a broad differential due to multisystemic involvement and the absence of specific clinical features.[64]Schutze GE, Jacobs RF. Human monocytic ehrlichiosis in children. Pediatrics. 1997 Jul;100(1):E10.
http://www.ncbi.nlm.nih.gov/pubmed/9200384?tool=bestpractice.com
[65]Schutze GE. Ehrlichiosis. Pediatr Infect Dis J. 2006 Jan;25(1):71-2.
http://www.ncbi.nlm.nih.gov/pubmed/16395107?tool=bestpractice.com
A presumptive diagnosis can be made in all patients with potential tick exposure/demonstrated tick bite combined with fever of abrupt onset, characteristic constitutional symptoms, leukopenia and/or thrombocytopenia, and elevated LFTs. Definitive diagnosis is confirmed by serology studies or polymerase chain reaction (PCR). A high index of suspicion is required for diagnosis of infection, even in endemic areas.[66]Weil AA, Baron EL, Brown CM, et al. Clinical findings and diagnosis in human granulocytic anaplasmosis: a case series from Massachusetts. Mayo Clin Proc. 2012 Mar;87(3):233-9.
http://www.ncbi.nlm.nih.gov/pubmed/22386178?tool=bestpractice.com
Ehrlichiosis and anaplasmosis are nationally notifiable diseases, and healthcare providers should notify local health departments, which in turn notify the State Health Department. The CDC is notified through the National Electronic Telecommunications System for Surveillance.
History
Potential tick exposure (e.g., outdoor activities in endemic areas during active tick months) and demonstrated tick bite within 14 days before onset of symptoms are key risk factors supporting a suspicion of any tick-borne disease. However, it is important to note that many patients do not have any recollection of a tick bite and therefore, lack of a tick bite history cannot exclude diagnosis in patients with signs, symptoms, and investigation results consistent with tick-borne illness.
[Figure caption and citation for the preceding image starts]: Tick bite at later stage with central necrosis (dark area around tick bite) surrounded by a markedly erythematous areaCourtesy of Lucas Blanton, MD [Citation ends].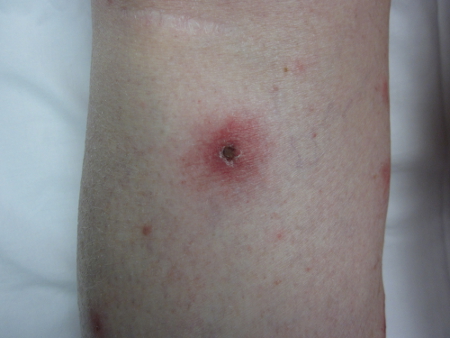
Infection typically presents as an acute illness; the incubation time is 1 to 2 weeks after tick bite. Some patients may be asymptomatic, although this is rare, especially in adults. Infection tends to be more severe in patients over 60 years of age.[16]Olano JP, Masters E, Hogrefe W, et al. Human monocytotropic ehrlichiosis, Missouri. Emerg Infect Dis. 2003 Dec;9(12):1579-86.
http://www.ncbi.nlm.nih.gov/pubmed/14720399?tool=bestpractice.com
[61]Fishbein DB, Kemp A, Dawson JE, et al. Human ehrlichiosis: prospective active surveillance in febrile hospitalized patients. J Infect Dis. 1989 Nov;160(5):803-9.
http://www.ncbi.nlm.nih.gov/pubmed/2809255?tool=bestpractice.com
[62]Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1989 Nov;160(5):803-9.
http://www.ncbi.nlm.nih.gov/pubmed/8604172?tool=bestpractice.com
[63]Horowitz HW, Aguero-Rosenfeld ME, McKenna DF, et al. Clinical and laboratory spectrum of culture-proven human granulocytic ehrlichiosis: comparison with culture-negative cases. Clin Infect Dis. 1998 Nov;27(5):1314-7.
http://www.ncbi.nlm.nih.gov/pubmed/9827289?tool=bestpractice.com
Fever of abrupt onset in combination with other constitutional symptoms such as rigors, myalgia, malaise, headache, arthralgia, or nausea is the most common presentation. Diagnosis should be considered in any patient with abrupt onset fever and a potential tick exposure. Less common presentations include nonspecific symptoms such as abdominal pain, vomiting, diarrhea, cough, dyspnea, and rash. Rash is more common in children than in adults. [Figure caption and citation for the preceding image starts]: Erythematous macular rash involving the lower extremity in a pediatric case of human monocytotropic/monocytic ehrlichiosisCourtesy of Edwin Masters, MD [Citation ends].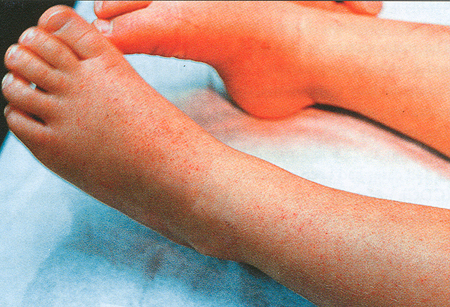
Neurologic symptoms such as stiff neck, photophobia, and confusion are rare.
Human granulocytotropic/granulocytic anaplasmosis (HGA) can coexist with Lyme disease, babesiosis, and tick-borne encephalitis.
Severe manifestations, such as hemophagocytic lymphohistiocytosis (HLH), a rare and potentially fatal immunologic syndrome, have also been reported in ehrlichiosis.[13]Otrock ZK, Gonzalez MD, Eby CS. Ehrlichia-induced hemophagocytic lymphohistiocytosis: a case series and review of literature. Blood Cells Mol Dis. 2015 Oct;55(3):191-3.
http://www.ncbi.nlm.nih.gov/pubmed/26227842?tool=bestpractice.com
[14]Patel TP, Beck P, Chairman D, et al. Ehrlichiosis presenting as hemophagocytic lymphohistiocytosis in an immunocompetent adult. IDCases. 2020;20:e00813.
https://www.sciencedirect.com/science/article/pii/S2214250920301219?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/32455115?tool=bestpractice.com
Signs and symptoms include hepatosplenomegaly, fever, and rash.
Physical exam
Typically, there are no findings on physical exam; however, a small round erythematous lesion with or without a small necrotic dark center may be seen on the skin (tick bite). Patients with human monocytotropic/monocytic ehrlichiosis (HME) may develop lymphadenopathy, hepatomegaly (more common in children), jaundice, or splenomegaly; however, this is rare. Central nervous system complications such as stupor, seizures, or coma are also rare but more common in HME than in HGA. Signs of candidiasis, cytomegalovirus infection, and aspergillosis may be seen in severe cases of HME and HGA. It is not fully understood why these patients are more susceptible but it is thought the acute infection with HME or HGA results in a level of immunosuppression.
Laboratory tests
CBC, peripheral blood smear, and LFTs should be ordered initially. Leukopenia with absolute and relative lymphopenia is common during the first week of the disease and is usually associated with thrombocytopenia; however, normal counts do not rule out the diagnosis. Cytoplasmic morulae are seen on blood smear, although this has not yet been observed in patients with Ehrlichia muriseauclairensis (formerly known as Ehrlichia muris-like agent, EMLA). Although this is a recommended investigation, detection of morulae is considered insensitive as a diagnostic test in immunocompetent patients. However, in clinical experience, in immunocompromised patients, a peripheral blood smear examination performed by an experienced hematologist may confirm the diagnosis more quickly.[67]Hamilton KS, Standaert SM, Kinney MC. Characteristic peripheral blood findings in human ehrlichiosis. Mod Pathol. 2004 May;17(5):512-7.
https://www.modernpathology.org/article/S0893-3952(22)04372-1/fulltext#seccestitle40
http://www.ncbi.nlm.nih.gov/pubmed/14976527?tool=bestpractice.com
Anemia is less frequent. LFTs are usually mildly to moderately elevated. Marked absolute and relative rebound lymphocytoses are seen during the second week of illness.[68]Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm Rep. 2016 May 13;65(2):1-44.
http://www.cdc.gov/mmwr/volumes/65/rr/rr6502a1.htm?s_cid=rr6502a1_w
http://www.ncbi.nlm.nih.gov/pubmed/27172113?tool=bestpractice.com
If LFTs are elevated and leukopenia and thrombocytopenia are present, this is very strong evidence for diagnosis. In the presence of strong suspicion, presumptive treatment would be started even if these tests are normal. Specific laboratory tests may then be pursued including serology or PCR testing. Specimens for diagnostic confirmatory tests should ideally be obtained before the patient receives the first dose of antibiotics.
Serology
Immunofluorescence antibody assay is the most definitive, widely available test for HME and HGA and is based on detection of a rise in antibodies against Ehrlichia chaffeensis or Anaplasma phagocytophilum in serum.[62]Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1989 Nov;160(5):803-9.
http://www.ncbi.nlm.nih.gov/pubmed/8604172?tool=bestpractice.com
[69]Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007 Jul 15;45 Suppl 1:S45-51.
http://www.ncbi.nlm.nih.gov/pubmed/17582569?tool=bestpractice.com
[70]Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000 Aug;31(2):554-60.
http://www.ncbi.nlm.nih.gov/pubmed/10987720?tool=bestpractice.com
[71]Olano J, Walker DH. Current recommendations for diagnosis and treatment of human ehrlichiosis. Infect Med. 2002;19:318-325.[72]Aguero-Rosenfeld ME, Horowitz HW, Wormser GP, et al. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996 Dec 1;125(11):904-8.
http://www.ncbi.nlm.nih.gov/pubmed/8967671?tool=bestpractice.com
[73]Comer JA, Nicholson WL, Sumner JW, et al. Diagnosis of human ehrlichiosis by PCR assay of acute-phase serum. J Clin Microbiol. 1999 Jan;37(1):31-4.
https://journals.asm.org/doi/10.1128/JCM.37.1.31-34.1999
http://www.ncbi.nlm.nih.gov/pubmed/9854059?tool=bestpractice.com
[74]Comer JA, Nicholson WL, Olson JG, et al. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999 Mar;37(3):558-64.
https://journals.asm.org/doi/10.1128/JCM.37.3.558-564.1999
http://www.ncbi.nlm.nih.gov/pubmed/9986812?tool=bestpractice.com
[75]Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev. 2003 Jan;16(1):37-64.
https://journals.asm.org/doi/10.1128/CMR.16.1.37-64.2003
http://www.ncbi.nlm.nih.gov/pubmed/12525424?tool=bestpractice.com
[76]Walker DH. Task Force on Consensus Approach for Ehrlichiosis. Diagnosing human ehrlichioses: current status and recommendations. ASM News. 2000;66:287-290.[77]Olano JP, Walker DH. Human ehrlichioses. Med Clin North Am. 2002 Mar;86(2):375-92.
http://www.ncbi.nlm.nih.gov/pubmed/11982308?tool=bestpractice.com
[78]Bakken JS, Haller I, Riddell D, et al. The serological response of patients infected with the agent of human granulocytic ehrlichiosis. Clin Infect Dis. 2002;34:22-27.
http://www.ncbi.nlm.nih.gov/pubmed/11731941?tool=bestpractice.com
[79]Walls JJ, Aguero-Rosenfeld M, Bakken JS, et al. Inter- and intralaboratory comparison of Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent strains for serodiagnosis of HGE by the immunofluorescent-antibody test. J Clin Microbiol. 1999 Sep;37(9):2968-73.
https://journals.asm.org/doi/10.1128/JCM.37.9.2968-2973.1999
http://www.ncbi.nlm.nih.gov/pubmed/10449483?tool=bestpractice.com
Paired samples should be obtained during the acute and convalescent phases (i.e., 2-4 weeks later) to demonstrate rising antibody titers. A fourfold or greater increase in antibody titre is diagnostic.[68]Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm Rep. 2016 May 13;65(2):1-44.
http://www.cdc.gov/mmwr/volumes/65/rr/rr6502a1.htm?s_cid=rr6502a1_w
http://www.ncbi.nlm.nih.gov/pubmed/27172113?tool=bestpractice.com
Serology cannot be used for diagnosis of human ewingii ehrlichiosis (HEE) due to extensive cross-reactive antibodies with E chaffeensis.
PCR
Detection of bacterial DNA/RNA from peripheral blood/tissue specimens using primers targeting a specific ehrlichial gene is the most definitive diagnostic finding for HME and HGA during the acute phase of the disease, when the patient is febrile and symptomatic.[16]Olano JP, Masters E, Hogrefe W, et al. Human monocytotropic ehrlichiosis, Missouri. Emerg Infect Dis. 2003 Dec;9(12):1579-86.
http://www.ncbi.nlm.nih.gov/pubmed/14720399?tool=bestpractice.com
[17]Standaert SM, Yu T, Scott MA, et al. Primary isolation of Ehrlichia chaffeensis from patients with febrile illnesses: clinical and molecular characteristics. J Infect Dis. 2000 Mar;181(3):1082-8.
http://www.ncbi.nlm.nih.gov/pubmed/10720534?tool=bestpractice.com
[19]Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995 Aug 17;333(7):420-5.
http://www.nejm.org/doi/full/10.1056/NEJM199508173330704#t=article
http://www.ncbi.nlm.nih.gov/pubmed/7616991?tool=bestpractice.com
[24]Bakken JS, Goellner P, Van Etten M, et al. Seroprevalence of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin Infect Dis. 1998 Dec;27(6):1491-6.
http://www.ncbi.nlm.nih.gov/pubmed/9868666?tool=bestpractice.com
[58]Paddock CD, Folk SM, Shore GM, et al. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001 Nov 1;33(9):1586-94.
http://www.ncbi.nlm.nih.gov/pubmed/11568857?tool=bestpractice.com
[62]Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1989 Nov;160(5):803-9.
http://www.ncbi.nlm.nih.gov/pubmed/8604172?tool=bestpractice.com
[72]Aguero-Rosenfeld ME, Horowitz HW, Wormser GP, et al. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996 Dec 1;125(11):904-8.
http://www.ncbi.nlm.nih.gov/pubmed/8967671?tool=bestpractice.com
[80]Everett ED, Evans KA, Henry RB, et al. Human ehrlichiosis in adults after tick exposure: diagnosis using polymerase chain reaction. Ann Intern Med. 1994 May 1;120(9):730-5.
http://www.ncbi.nlm.nih.gov/pubmed/8147545?tool=bestpractice.com
[81]Anderson BE, Sumner JW, Dawson JE, et al. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992 Apr;30(4):775-80.
https://journals.asm.org/doi/10.1128/jcm.30.4.775-780.1992
http://www.ncbi.nlm.nih.gov/pubmed/1374076?tool=bestpractice.com
[82]Sirigireddy KR, Ganta RR. Multiplex detection of Ehrlichia and Anaplasma species pathogens in peripheral blood by real-time reverse transcriptase-polymerase chain reaction. J Mol Diagn.2005 May;7(2):308-16.
http://www.ncbi.nlm.nih.gov/pubmed/15858156?tool=bestpractice.com
[83]Doyle CK, Labruna MB, Breitschwerdt EB, et al. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn.2005 Oct;7(4):504-10.
http://www.ncbi.nlm.nih.gov/pubmed/16237220?tool=bestpractice.com
[84]Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci. 2006 Oct;1078:236-47.
http://www.ncbi.nlm.nih.gov/pubmed/17114714?tool=bestpractice.com
[85]Bakken JS, Dumler JS, Chen SM, et al. Human granulocytic ehrlichiosis in the upper Midwest United States: a new species emerging? JAMA. 1994 Jul 20;272(3):212-8.
http://www.ncbi.nlm.nih.gov/pubmed/8022040?tool=bestpractice.com
[86]Courtney JW, Dryden RL, Wyleto P, et al. Characterization of Anaplasma phagocytophila and Borrelia burgdorferi genotypes in Ixodes scapularis ticks from Pennsylvania. Ann N Y Acad Sci. 2003 Jun;990:131-3.
http://www.ncbi.nlm.nih.gov/pubmed/12860614?tool=bestpractice.com
[87]Dumler JS, Brouqui P. Molecular diagnosis of human granulocytic anaplasmosis. Expert Rev Mol Diagn. 2004 Jul;4(4):559-69.
http://www.ncbi.nlm.nih.gov/pubmed/15225103?tool=bestpractice.com
PCR using primers specific for E ewingii is the only method available for diagnosing HEE;E ewingii is not culturable and serologic cross-reactions with E chaffeensis are extensive.[4]Buller RS, Arens M, Hmiel SP, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999 Jul 15;341(3):148-55.
http://www.nejm.org/doi/full/10.1056/NEJM199907153410303#t=article
http://www.ncbi.nlm.nih.gov/pubmed/10403852?tool=bestpractice.com
[88]Gusa AA, Buller RS, Storch GA, et al. Identification of a p28 gene in Ehrlichia ewingii: evaluation of gene for use as a target for a species-specific PCR diagnostic assay. J Clin Microbiol. 2001 Nov;39(11):3871-6.
http://www.ncbi.nlm.nih.gov/pubmed/11682500?tool=bestpractice.com
Other tests used to confirm diagnosis include Western immunoblotting, culture, and immunohistochemistry; however, these tests are not routinely used.
Coinfections
Infections with Borrelia spp. and Babesia spp. can occur simultaneously or sequentially in cases of HGA due to the presence of the first 2 pathogens in Ixodes ticks.[89]Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001 Sep 1;33(5):676-85.
http://www.ncbi.nlm.nih.gov/pubmed/11486290?tool=bestpractice.com
[90]Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996 Jun 5;275(21):1657-60.
http://www.ncbi.nlm.nih.gov/pubmed/8637139?tool=bestpractice.com
[91]Benach JL, Coleman JL, Habicht GS, et al. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. J Infect Dis. 1985 Sep;152(3):473-7.
http://www.ncbi.nlm.nih.gov/pubmed/4031555?tool=bestpractice.com
[92]Magnarelli LA, Dumler JS, Anderson JF, et al. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J Clin Microbiol. 1995 Nov;33(11):3054-7.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC228637
http://www.ncbi.nlm.nih.gov/pubmed/8576376?tool=bestpractice.com
[93]Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997 Jul 3;337(1):27-30.
http://www.nejm.org/doi/full/10.1056/NEJM199707033370105
http://www.ncbi.nlm.nih.gov/pubmed/9203428?tool=bestpractice.com
In the northeast and upper midwest areas of the US, the vector is Ixodes scapularis, whereas in the western part of the country the vector is I pacificus. The incidence of borreliosis and babesiosis is lower in the latter.[89]Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001 Sep 1;33(5):676-85.
http://www.ncbi.nlm.nih.gov/pubmed/11486290?tool=bestpractice.com
The natural reservoir for all 3 pathogens is the white-footed mouse (Peromyscus leucopus), whereas deer (Odocoileus virginianus or white-tailed deer) are the definitive hosts of these tick vectors. Coinfections in Europe and eastern Europe/Asia also occur but the vectors are I ricinus and I persulcatus, respectively.[26]Heyman P, Cochez C, Hofhuis A, et al. A clear and present danger: tick-borne diseases in Europe. Exp Rev Anti Infect Ther. 2010 Jan;8(1):33-50.
http://www.ncbi.nlm.nih.gov/pubmed/20014900?tool=bestpractice.com
The presence of more than 1 of these pathogens in the vectors varies greatly depending on the geographic area studied.[89]Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001 Sep 1;33(5):676-85.
http://www.ncbi.nlm.nih.gov/pubmed/11486290?tool=bestpractice.com
[94]Mather TN, Telford SR 3rd, Moore SI, et al. Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini). Exper Parasitol. 1990 Jan;70(1):55-61.
http://www.ncbi.nlm.nih.gov/pubmed/2295326?tool=bestpractice.com
The pathogens responsible for borreliosis in the US and Europe include B burgdorferi sensu stricto, B garini (higher frequency of neurologic disease), and B afzelii. The latter 2 are more prevalent in Europe.[26]Heyman P, Cochez C, Hofhuis A, et al. A clear and present danger: tick-borne diseases in Europe. Exp Rev Anti Infect Ther. 2010 Jan;8(1):33-50.
http://www.ncbi.nlm.nih.gov/pubmed/20014900?tool=bestpractice.com
[95]Aguero-Rosenfeld ME. Laboratory aspects of tick-borne diseases: lyme, human granulocytic ehrlichiosis and babesiosis. Mt Sinai J Med. 2003 May;70(3):197-206.
http://www.ncbi.nlm.nih.gov/pubmed/12764539?tool=bestpractice.com
[96]van Dam AP, Kuiper H, Vos K, et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993 Oct;17(4):708-17.
http://www.ncbi.nlm.nih.gov/pubmed/7903558?tool=bestpractice.com
As for babesiosis, most infections in the US are caused by B microti (northeast and midwest). B duncaniand the unnamed species CA-1 occur toward the west coast of the US, while MO-1 occurs in Missouri.[89]Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001 Sep 1;33(5):676-85.
http://www.ncbi.nlm.nih.gov/pubmed/11486290?tool=bestpractice.com
[97]Conrad PA, Kjemtrup AM, Carreno RA, et al. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006 Jun;36(7):779-89.
http://www.ncbi.nlm.nih.gov/pubmed/16725142?tool=bestpractice.com
[98]Centers for Disease Control and Prevention. Babesiosis. Jun 2024 [internet publication].
https://www.cdc.gov/dpdx/babesiosis/index.html
In Europe, the main pathogen is B divergens.[26]Heyman P, Cochez C, Hofhuis A, et al. A clear and present danger: tick-borne diseases in Europe. Exp Rev Anti Infect Ther. 2010 Jan;8(1):33-50.
http://www.ncbi.nlm.nih.gov/pubmed/20014900?tool=bestpractice.com
Diagnoses of coinfections should rely on direct methods such as microscopic visualization of the pathogen (spirochetes seen by silver stains from skin biopsies, PCR, or culture in patients with borreliosis; intraerythrocitic parasites visualized in peripheral blood smears and PCR in cases of babesiosis; and microscopic visualization of intracellular morulae in polymorphonuclear leukocytes from peripheral blood smears, PCR, or culture in cases of HGA).[95]Aguero-Rosenfeld ME. Laboratory aspects of tick-borne diseases: lyme, human granulocytic ehrlichiosis and babesiosis. Mt Sinai J Med. 2003 May;70(3):197-206.
http://www.ncbi.nlm.nih.gov/pubmed/12764539?tool=bestpractice.com
Serologic methods should be avoided for diagnosis of coinfections due to high rate of antibodies against these agents in highly endemic areas. However, evidence of rising antibodies in samples taken 2 to 4 weeks apart strongly suggest the presence of an acute infection by any of those pathogens. Clinically, the diagnosis of B burgdorferi infections is relatively easy if the classic skin manifestations are present such as erythema migrans or annular rash. However, both HGA and babesiosis present as undifferentiated febrile illnesses with no distinguishing features to differentiate them from each other or other nonspecific febrile illnesses including tick-borne diseases such as Rocky Mountain spotted fever and tick-borne encephalitis (caused by flaviviruses). The clinical manifestations of both Lyme disease and babesiosis are usually more severe when compared with infections that occur separately.[89]Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001 Sep 1;33(5):676-85.
http://www.ncbi.nlm.nih.gov/pubmed/11486290?tool=bestpractice.com
[90]Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996 Jun 5;275(21):1657-60.
http://www.ncbi.nlm.nih.gov/pubmed/8637139?tool=bestpractice.com

