NSVT is by definition a self-terminating event, and therefore usually no specific treatment is indicated. Rather, treatment is directed at any existing heart condition. Patients with underlying cardiac disease may need more aggressive therapy due to increased mortality risk. In contrast, no increase in mortality has been demonstrated in those patients without an associated cardiac condition; therefore, reassurance is usually sufficient.
In asymptomatic patients, if investigations show that the NSVT is likely to be idiopathic (right ventricular outflow tract or fascicular origin, negative family history, normal 12-lead ECG and echocardiogram), and the NSVT/premature ventricular contraction (PVC) burden is less than 10%, the patient can be discharged and re-evaluated if they experience new symptoms or changes in their clinical condition.[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Evaluation of nonsustained wide QRS tachycardiaCreated by BMJ Knowledge Centre. [Citation ends].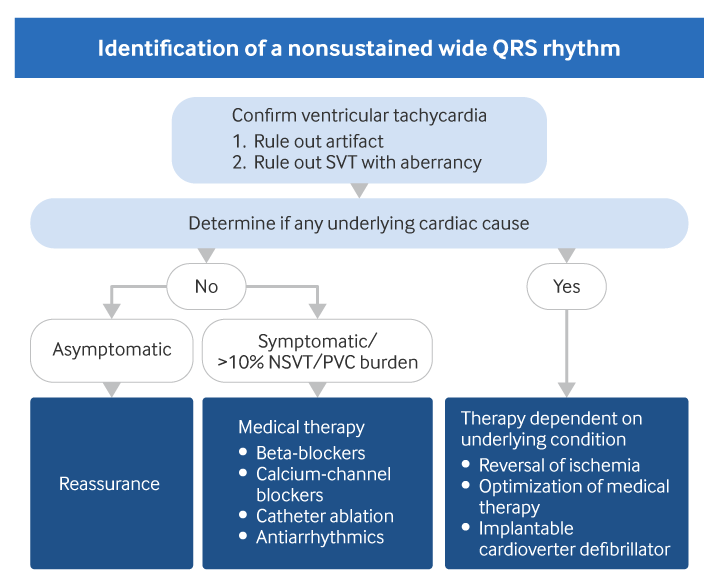
Modifiable risk factors for coronary artery disease should be addressed to prevent myocardial infarction (MI) and left ventricular dysfunction. Necessary medical therapy depending on the underlying condition should be undertaken first before more aggressive treatment such as an implantable device is considered. It should also be noted that, although there are international differences regarding the use of therapies such as implantable cardioverter defibrillators (ICDs), the guideline for the prevention of sudden cardiac death due to ventricular arrhythmias was developed jointly by European and US professional cardiology groups.[1]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018 Sep 25;138(13):e272-e391.
https://www.doi.org/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
Symptomatic NSVT or >10% asymptomatic NSVT/PVC burden
On the rare occasion that NSVT produces symptoms in the absence of cardiac disease, medication or catheter ablation may be required.[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Symptom correlation with the NSVT is necessary and usually accomplished by ambulatory ECG monitoring. Medical treatment options for symptomatic or high-burden NSVT include beta-blockers or calcium-channel blockers (usually used when beta-blockers are contraindicated, e.g., asthma) as first-line therapy.[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Catheter ablation may be considered early in the treatment course and has a high success rate, particularly with right ventricular outflow tract and fascicular origin NSVT, where it is recommended as first-line treatment.[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Antiarrhythmics such as flecainide or propafenone may be used in patients who fail therapy with beta-blockers and/or calcium-channel blockers who are not candidates for catheter ablation or in whom catheter ablation is ineffective. Amiodarone should only be used if ablation or other medications fail or cannot be used, due to its toxicity.[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
The far more common clinical scenario requiring treatment is when NSVT is associated with a high burden of PVCs, and reduced left ventricular function is present. Frequent ventricular ectopy (NSVT and PVCs) can sometimes be a causal factor for reduced left ventricular function, or an important contributor.[39]Gorenek B, Fisher JD, Kudaiberdieva G, et al. Premature ventricular complexes: diagnostic and therapeutic considerations in clinical practice : A state-of-the-art review by the American College of Cardiology Electrophysiology Council. J Interv Card Electrophysiol. 2020 Jan;57(1):5-26.
http://www.ncbi.nlm.nih.gov/pubmed/31828560?tool=bestpractice.com
The reasons for this are multifactorial but dyssynchronous left ventricular depolarization is an important mechanistic cause. Although a 10% PVC burden is often used clinically as the percentage of ventricular ectopy that may lead to left ventricular dysfunction, it is important to know that the burden of ventricular ectopy can have temporal variability, and extended monitoring may be required.[40]Mullis AH, Ayoub K, Shah J, et al. Fluctuations in premature ventricular contraction burden can affect medical assessment and management. Heart Rhythm. 2019 Oct;16(10):1570-74.
http://www.ncbi.nlm.nih.gov/pubmed/31004780?tool=bestpractice.com
As a corollary, many patients with a PVC burden >10% will not develop left ventricular dysfunction, and in 44% of patients, PVCs will spontaneously resolve at 1 to 2 year follow-up, so treatment to reduce PVC burden should only be considered in an asymptomatic patient if left ventricular dysfunction is present.[41]Lee AKY, Andrade J, Hawkins NM, et al. Outcomes of untreated frequent premature ventricular complexes with normal left ventricular function. Heart. 2019 Sep;105(18):1408-13.
http://www.ncbi.nlm.nih.gov/pubmed/31142596?tool=bestpractice.com
However, in the setting of left ventricular dysfunction and persistent PVC burden >10% despite treatment of reversible conditions, a trial of antiarrhythmic drugs, or referral for catheter ablation, should be considered to determine whether left ventricular function improves with reduction in PVC burden.[1]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018 Sep 25;138(13):e272-e391.
https://www.doi.org/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
Electrolyte disturbances
Electrolyte abnormalities, most commonly hypokalemia, hyperkalemia, and hypomagnesemia, may trigger NSVT in patients with or without cardiac disease. These disturbances should be corrected as efficiently as possible.
Chronic coronary artery disease (CAD)
Patients with NSVT and CAD, combined with a decreased left ventricular ejection fraction (LVEF) (≤40%), are at high risk of death from cardiac causes.[1]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018 Sep 25;138(13):e272-e391.
https://www.doi.org/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
[42]Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 Dec 16;341(25):1882-90.
https://www.nejm.org/doi/10.1056/NEJM199912163412503?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/10601507?tool=bestpractice.com
In these patients, medical therapy should be optimized and modifiable risk factors should be addressed, including weight management, physical activity, and smoking cessation. Electrophysiologic testing is indicated for risk stratification. In patients with sustained inducible VT, ICD implantation has been shown to reduce the risk of death and cardiac arrest independently of New York Heart Association class.[1]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018 Sep 25;138(13):e272-e391.
https://www.doi.org/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[42]Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 Dec 16;341(25):1882-90.
https://www.nejm.org/doi/10.1056/NEJM199912163412503?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/10601507?tool=bestpractice.com
Post-MI
Early reperfusion decreases overall prevalence of NSVT following acute MI.[9]Maggioni AP, Zuanetti G, Franzosi MG, et al. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. Circulation. 1993 Feb;87(2):312-22.
http://circ.ahajournals.org/cgi/reprint/87/2/312
http://www.ncbi.nlm.nih.gov/pubmed/8093865?tool=bestpractice.com
Optimization of medical therapy including beta-blockers, ACE inhibitors, antiplatelet therapy, and statins, reduces the risk of sudden cardiac death following acute MI.[43]Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998 Nov 5;339(19):1349-57.
http://content.nejm.org/cgi/content/full/339/19/1349
http://www.ncbi.nlm.nih.gov/pubmed/9841303?tool=bestpractice.com
[44]Levantesi G, Scarano M, Marfisi R, et al. Metaanalysis of effect of statin treatment on risk of sudden death. Am J Cardiol. 2007 Dec 1;100(11):1644-50.
http://www.ncbi.nlm.nih.gov/pubmed/18036362?tool=bestpractice.com
[45]Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981 Apr 2;304(14):801-7.
http://www.ncbi.nlm.nih.gov/pubmed/7010157?tool=bestpractice.com
[46]Beta Blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. I: mortality results. JAMA. 1982 Mar 26;247(12):1707-14.
http://www.ncbi.nlm.nih.gov/pubmed/7038157?tool=bestpractice.com
[47]Makikallio TH, Barthel P, Schneider R, et al. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am J Cardiol. 2006 Feb 15;97(4):480-4.
http://www.ncbi.nlm.nih.gov/pubmed/16461041?tool=bestpractice.com
[48]Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309-21.
http://www.nejm.org/doi/full/10.1056/NEJMoa030207#t=article
http://www.ncbi.nlm.nih.gov/pubmed/12668699?tool=bestpractice.com
[49]He XZ, Zhou SH, Wan XH, et al. The effect of early and intensive statin therapy on ventricular premature beat or nonsustained ventricular tachycardia in patients with acute coronary syndrome. Clin Cardiol. 2011 Jan;34(1):59-63.
http://www.ncbi.nlm.nih.gov/pubmed/21259280?tool=bestpractice.com
In patients who have symptomatic NSVT following MI, amiodarone may be beneficial in reducing risk of sudden cardiac death.[50]Cairns JA, Connolly SJ, Roberts R, et al. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Lancet. 1997 Mar 8;349(9053):675-82.
http://www.ncbi.nlm.nih.gov/pubmed/9078198?tool=bestpractice.com
However, several randomized trials have found that amiodarone is not associated with improved clinical outcomes. In addition, the many adverse effects associated with amiodarone limit its use in many clinical situations; therefore it is generally not used.
Modifiable risk factors such as obesity and smoking should also be addressed with a carefully planned exercise, diet, and weight loss program to further reduce the incidence of sudden cardiac death.[51]Kannel WB, Thomas HE Jr. Sudden coronary death. the Framingham Study. Ann N Y Acad Sci. 1982 Mar;382(1):3-21.
http://www.ncbi.nlm.nih.gov/pubmed/7044245?tool=bestpractice.com
[52]Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004 May 15;116(10):682-92.
http://www.ncbi.nlm.nih.gov/pubmed/15121495?tool=bestpractice.com
ICD is recommended for patients after MI with NSVT, ejection fraction (EF) ≤40% and inducible ventricular arrhythmias at electrophysiologic testing.[53]Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Circulation. 2008 Jun;117(6):e350-408.
http://circ.ahajournals.org/cgi/content/full/117/21/e350
http://www.ncbi.nlm.nih.gov/pubmed/18483207?tool=bestpractice.com
Routine early use of ICD is not recommended.[54]Steinbeck G, Andresen D, Seidl K, et al; IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009 Oct 8;361(15):1427-36.
http://www.ncbi.nlm.nih.gov/pubmed/19812399?tool=bestpractice.com
Heart failure after MI
Medications such as beta-blockers, ACE inhibitors, and aldosterone receptor antagonists reduce overall mortality and incidence of sudden cardiac death in patients with heart failure.[48]Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309-21.
http://www.nejm.org/doi/full/10.1056/NEJMoa030207#t=article
http://www.ncbi.nlm.nih.gov/pubmed/12668699?tool=bestpractice.com
[55]SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991 Aug 1;325(5):293-302.
http://www.ncbi.nlm.nih.gov/pubmed/2057034?tool=bestpractice.com
[56]CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): a randomised trial. Lancet. 1999 Jan 2;353(9146):9-13.
http://www.ncbi.nlm.nih.gov/pubmed/10023943?tool=bestpractice.com
[57]Wei J, Ni J, Huang D, et al. The effect of aldosterone antagonists for ventricular arrhythmia: a meta-analysis. Clin Cardiol. 2010 Sep;33(9):572-7.
http://www.ncbi.nlm.nih.gov/pubmed/20842742?tool=bestpractice.com
Diuretics have no effect on mortality and are usually indicated to help relieve symptoms of fluid overload.
ICD is recommended for patients who are at least 40 days post-MI with class II/III heart failure, LVEF ≤35%, and who have a reasonable expectation of meaningful survival for >1 year.[58]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-e1032.
https://www.ahajournals.org/doi/reader/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
ICD is similarly indicated in patients with class I heart failure if LVEF is ≤30%.[58]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-e1032.
https://www.ahajournals.org/doi/reader/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Comorbid conditions that may attenuate the survival benefit of ICDs include chronic kidney disease, diabetes, peripheral vascular disease, and elevated BUN (27 to 50 mg/dL). It is important to note that the decision to implant an ICD is based on cardiac function and symptoms rather than the presence or absence of NSVT.[53]Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Circulation. 2008 Jun;117(6):e350-408.
http://circ.ahajournals.org/cgi/content/full/117/21/e350
http://www.ncbi.nlm.nih.gov/pubmed/18483207?tool=bestpractice.com
In some patients with heart failure, particularly those at risk for sudden cardiac death and who also have discordant contraction of left ventricular function, a cardiac resynchronization therapy (CRT) device can be used, usually in combination with an ICD (CRT-D).[59]Ketha S, Kusumoto F. Cardiac resynchronization therapy in 2015: lessons learned. Cardiovasc Innov Applications. 2015 Oct;1(1):93-106
https://www.scienceopen.com/hosted-document?doi=10.15212/CVIA.2015.0011
The CRT device is designed to improve cardiac function by providing more coordinated contraction of the left ventricle. However, frequent episodes of NSVT or frequent PVCs can interfere with the normal function of the device.
Idiopathic or hypertrophic cardiomyopathy
ICD placement is recommended for patients with underlying idiopathic cardiomyopathy plus LVEF ≤35% and class II/III heart failure.[1]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018 Sep 25;138(13):e272-e391.
https://www.doi.org/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
ICD is not indicated for patients with NSVT and idiopathic cardiomyopathy with good left ventricular function.
European Society of Cardiology guidelines recommend that ICD placement be considered in patients with NSVT and cardiomyopathy who meet the following criteria:[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Dilated cardiomyopathy/hypokinetic nondilated cardiomyopathy, a pathogenic mutation in the LMNA gene, and a 5-year estimated risk of life-threatening ventricular arrhythmia of ≥10%.
Symptomatic arrhythmogenic right ventricular cardiomyopathy and moderate right ventricle (<40%) and/or left ventricle (<45%) dysfunction.
NSVT is found on 24-hour ambulatory ECG monitoring in 20% to 25% of patients with hypertrophic cardiomyopathy (HCM), and is associated with increasing left ventricular wall thickness and late gadolinium enhancement on cardiac magnetic resonance imaging. The relationship between NSVT and HCM prognosis is not yet clear.[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
ICD placement should be considered for patients with HCM who have one of the major risk factors for sudden cardiac death. Risk factors include sudden cardiac arrest, spontaneous sustained VT, unexplained syncope, family history of sudden cardiac death in a first-degree relative, ventricular septal wall thickness greater than 30 mm, NSVT on 24-hour ambulatory ECG monitoring, and hypotension in response to exercise. Other risk factors include atrial fibrillation, myocardial ischemia, left ventricular outflow tract obstruction, a high-risk mutation, and patients who compete in high-intensity physical exercises.[1]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018 Sep 25;138(13):e272-e391.
https://www.doi.org/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
[2]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
If patients have an identified genetic mutation but there are no overt signs of the disease and no risk factors for sudden cardiac death, close observation is sufficient; activity restrictions are not required.[60]Maron BJ, Ackerman MJ, Nishimura RA, et al. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005 Apr 19;45(8):1340-5.
http://content.onlinejacc.org/article.aspx?articleID=1136515
http://www.ncbi.nlm.nih.gov/pubmed/15837284?tool=bestpractice.com
