Tests
1st tests to order
Tests to consider
esophagogastroduodenoscopy (EGD)
Test
Indicated for alarm symptoms or symptoms suggesting complicated disease (atypical, persistent, or relapsing symptoms).[1][5][36][37][Figure caption and citation for the preceding image starts]: Moderate to severe esophagitis with multiple linear, clean-based esophageal ulcersFrom the collection of Dr Douglas G. Adler; used with permission [Citation ends].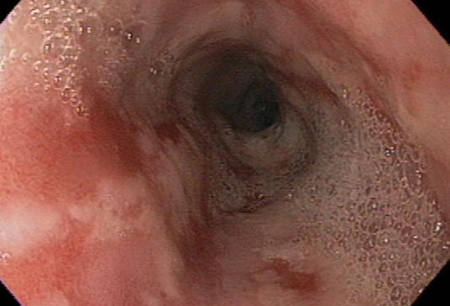
Consider evaluation for nongastrointestinal causes before endoscopy in patients with isolated extraesophageal features (e.g., laryngitis, globus, tooth enamel erosion, halitosis).[6]
PPIs should be stopped 2-4 weeks before the procedure.[1]
Normal mucosa is the most common finding in patients with typical GERD symptoms.[1] Routine biopsies are not recommended if there are no clinical and/or endoscopic features suggestive of eosinophilic esophagitis or Barrett esophagus.[37][45] Biopsies should be performed if endoscopy is performed for refractory GERD, even if the mucosa appears normal.[1]
Evidence is conflicting as to whether the frequency and severity of symptoms can predict Barrett esophagus, severity of esophagitis, or other complications. Healing of higher grades of erosive esophagitis may be associated with the finding of Barrett esophagus. Thus, if endoscopy is performed because of concern for Barrett esophagus (e.g., longstanding symptoms), it may be best to carry out the procedure after an 8-week course of proton-pump inhibitor treatment.[37]
Result
normal or may show esophagitis (erosion, ulcerations, strictures) or Barrett esophagus
ambulatory pH monitoring
Test
Can demonstrate abnormal exposure to esophageal acid in the absence of esophagitis.[46]
There are two types of pH monitoring: nasoesophageal catheter and wireless radiotelemetry capsule monitoring. Wireless radiotelemetry allows monitoring for 48 hours without a nasoesophageal catheter, which results in less discomfort and fewer interruptions of daily activities.
Either ambulatory pH or impedance-pH is recommended to evaluate patients with a suspected esophageal GERD syndrome who have not responded to an empiric trial of PPI therapy, have normal findings on endoscopy, and have no major motor abnormality on manometry.[1][3]
Controversy exists whether to perform pH monitoring while on or off PPI therapy. AGA guidance recommends ambulatory pH or impedance-pH monitoring while off PPI therapy for 7 days.[3][36] The Lyon Consensus recommends pH testing off PPI therapy for patients with no (or low-grade) esophagitis at endoscopy and no prior positive pH testings.[47]
To measure nonacid reflux, ACG guidelines recommend impedance-pH monitoring while on PPI therapy.[1]
Result
pH <4 more than 4% of the time is abnormal
esophageal manometry
Test
Manometry evaluates esophageal contractions and lower esophageal sphincter function. It may detect subtle presentations of esophageal motility disorders such as achalasia or diffuse esophageal spasm.
According to the American Gastroenterological Association guidelines, motility abnormalities associated with GERD can be detected using high-resolution esophageal manometry; however, manometry should not be used as the sole diagnostic test.[1]
The American College of Gastroenterology advises that manometry is performed before antireflux surgery, in patients unresponsive to PPIs in whom impedance-pH monitoring has not determined an etiology, and in patients with noncardiac chest pain, especially those who have not responded to a trial of PPIs.[1]
Result
may suggest achalasia, esophageal spasm, or other motor disorders
combined impedance-pH testing
Test
Esophageal impedance testing detects antegrade and retrograde bolus transit of liquid and gas. Impedance monitoring cannot detect the acid content or volume of the intraluminal contents. Therefore, a pH probe is usually incorporated into the assembly.
Combined impedance-pH monitoring can thus detect acid as well as nonacid reflux, to assess correlation with symptoms.[3][48]
Either ambulatory pH or impedance-pH is recommended to evaluate patients with a suspected esophageal GERD syndrome who have not responded to an empiric trial of PPI therapy, have normal findings on endoscopy, and have no major motor abnormality on manometry.[1][3]
Controversy exists whether to perform pH monitoring while on or off PPI therapy. AGA guidance recommends ambulatory pH or impedance-pH monitoring while off PPI therapy for 7 days.[3][36]
To measure nonacid reflux, ACG guidelines recommend impedance-pH monitoring while on PPI therapy.[1]
Outcome studies evaluating usefulness are needed for impedance-pH testing in investigation of refractory reflux symptoms.[49][50]
Result
may detect acid or nonacid reflux events
barium swallow
Test
A barium swallow may be useful in patients with dysphagia for whom endoscopy is contraindicated or unavailable.[41][42]
Barium imaging should not be used solely as a diagnostic test for GERD.[1] The presence of reflux on a barium esophagram has poor sensitivity and specificity for GERD, compared with pH testing.[1]
Result
may exclude other causes of dysphagia
esophageal capsule endoscopy
Test
Involves swallowing a capsule endoscope to visualize the esophagus. Capsule endoscopy does not require sedation.
A less-invasive alternative to upper endoscopy, and a potential screening and diagnostic tool to evaluate esophageal pathology. Studies have shown only moderate sensitivity and specificity for diagnosis of esophageal disorders, and it has a limited role and acceptance in screening for mucosal disease (erosive esophagitis and Barrett esophagus).[38][39][40]
Capsule endoscopy is done for patient convenience in select circumstances. It is contraindicated in the presence of suspected (e.g., presence of dysphagia) or known stricture or adhesions.
Result
may show esophagitis or Barrett esophagus
Emerging tests
endolumenal functional lumen imaging probe
Test
FDA approved catheter-based assessment that simultaneously measures the cross-sectional area, distensibility, and intraluminal pressure of the esophagus. Performed during upper endoscopy.
Due to limited evidence, the endolumenal functional lumen imaging probe is not currently recommended in routine GERD management.[51]
Result
Use of this content is subject to our disclaimer