Approach
Clinical diagnosis is confirmed with laboratory toxin identification.[41] Electrophysiologic testing should only be used if laboratory results are negative, but clinical suspicion of botulism is high or Lambert-Eaton myasthenic syndrome is suspected.
Risk factors associated with botulism should be sought and include ingestion of contaminated foods. A deliberate release of botulinum toxin (biologic terrorism) is strongly associated with the development of the condition and should also be considered. Other weaker risk factors include: ingestion of honey or soil in infants, contact with reptiles (specifically terrapins), intravenous drug use, crush injury, abnormal bowel anatomy, and therapeutic or cosmetic use of botulinum toxin.[39]
Clinical presentation
While botulinum toxin may reach the neuromuscular junction via several routes (foodborne, wound, iatrogenic, or inhalational), the clinical presentations of each are often indistinguishable.
Foodborne botulism
Characterized by bilateral cranial nerve palsies (blurred vision and diplopia due to paralysis of III, IV, and VI; dysarthria and dysphagia due to paralysis of IX, X, and XII) within 2 to 36 hours of ingestion of contaminated food, followed by symmetrical, descending flaccid paralysis.[42][43]
Abdominal cramps, nausea, vomiting, and diarrhea may also occur early in the illness, although these symptoms are often attributed to coincidental non-Clostridium pathogens.[44]
Affected patients are not febrile, confused, or obtunded, and the sensory system is spared.
Autonomic dysfunction may manifest as hypothermia, urinary retention, dry mouth and throat, postural hypotension, and constipation.
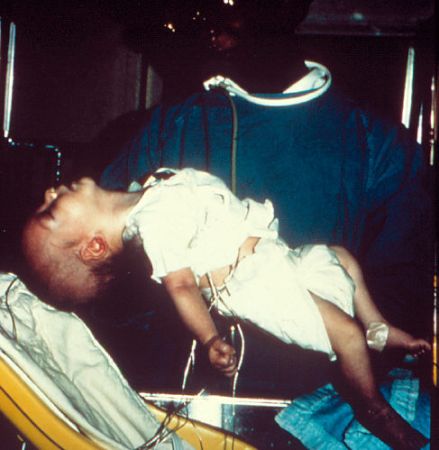
Infant botulism
Bulbar and extremity weakness follow as affected infants develop feeding difficulties, a weakened cry, ptosis, and hypotonia.[Figure caption and citation for the preceding image starts]: Six-week-old infant with botulismCDC [Citation ends].
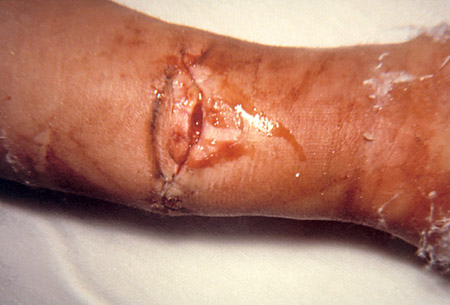
Wound botulism
Presents with neurologic findings identical to those of foodborne illness in the absence of a gastrointestinal prodrome, and the incubation period is longer (4-14 days).[21][Figure caption and citation for the preceding image starts]: Wound botulism involvement of compound fracture of right armCDC [Citation ends].
Inhalational botulism
The signs and symptoms exhibited are the same as those seen with foodborne illness.
The latency between exposure and clinical disease after inhalation appears to be between 12 hours and 5 days.[47]
Iatrogenic botulism
Presents with neurologic findings identical to those of foodborne illness in the absence of a gastrointestinal prodrome.
Physical exam
Early signs
Oculobulbar weakness.
Impaired accommodation and ptosis occur due to paralysis of cranial nerves III, IV, and VI.
Hypoglossal weakness is a sign of cranial nerve IX, X, and XII involvement.
Late signs
Descending symmetrical paralysis affecting the voluntary muscles of the neck, shoulders, and upper extremities, followed by the proximal and distal lower extremities.
Deep tendon reflexes, initially present, diminish or disappear within a few days of infection.
Respiratory dysfunction may result from either upper airway obstruction (pharyngeal collapse due to cranial nerve involvement) or diaphragmatic and accessory muscle weakness.
Pupillary dilation occurs in <50% of cases.[44] Its absence does not diminish the likelihood of botulism.
Laboratory evaluation
Conventional diagnosis of botulism relies on the demonstration of toxin in serum, gastric secretions, stool, or food samples.[41]
Mouse bioassay
Performed in all patients with suspected botulism.
The most sensitive means of botulism toxin detection.[48]
Serum, gastric secretions, stool, or food samples are diluted in a phosphate buffer and injected into the peritoneum of laboratory mice.
The mice are then followed for the development of botulism-like symptoms: fuzzy hair, muscle weakness, and respiratory failure.
Toxin type may be determined by injecting infected mice with type-specific botulism antitoxin. Botulism symptoms are absent from infected mice that receive the appropriate antitoxin. Confirmation and toxin typing are obtained in almost 75% of cases.[49]
Labor and resource intensive. Therefore, the testing is performed in a limited number of public health laboratories.
Testing should be performed under the direction of local state or health departments or the Centers for Disease Control and Prevention. CDC: Information for health professionals: botulism Opens in new window British Columbia Centre for Disease Control: laboratory services Opens in new window
A level 2 containment facility is a minimum requirement for Clostridium botulinum detection and evaluation, given its potency.
Culture
Performed only in cases of foodborne or infant botulism.
Culture of food samples, gastric aspirate, or fecal material from affected patients may reveal C botulinum.
Strict anaerobic conditions are required for growth, and competing fecal flora or nontoxigenic C botulinum strains may make isolation difficult.
Electrophysiologic testing
In patients with a clinical syndrome suggestive of botulism who have negative toxin assay and stool culture results, electrophysiologic testing can provide a presumptive diagnosis.[50]
Characterized by a small evoked muscle action potential in response to supramaximal nerve stimulus of a clinically affected muscle.
Sensory nerve studies are normal.
Motor conduction velocities are normal.
The amplitude of compound muscle action potentials is reduced in 85% of cases.
Repetitive nerve stimulation at high rates (≥20 Hz) may reveal a small increment in the motor response.
Post-tetanic facilitation (PTF) is 30% to 100% in botulism and may last for several minutes. In cases of Lambert-Eaton myasthenic syndrome (LEMS), PTF is 200% or more but lasts only 30 to 60 seconds.
This test is very uncomfortable and should not be requested unless botulism or LEMS is a serious consideration.
Emerging tests for foodborne botulism
Enzyme-linked immunosorbent assay
Has demonstrated botulinum toxin in contaminated food samples such as fish fillets, canned salmon and corned beef, pasta products, and canned vegetables.[51][52][53][54]
Polymerase chain reaction
May have a role as a rapid method of diagnosis; however, cellular components of clinical and food samples may limit sensitivity.[55]
Greater sensitivity may be achieved with extracted DNA, but the process may be laborious and time-consuming.
Use of this content is subject to our disclaimer