History and exam
Key diagnostic factors
common
acanthosis nigricans
Present in 90% to 95% of patients.[57]
A cutaneous manifestation of insulin resistance characterized by velvety, hyperpigmented skin, most often in the intertriginous areas. [Figure caption and citation for the preceding image starts]: Acanthosis nigricansFrom the collection of Dr Jennifer Miller [Citation ends].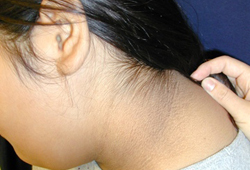 [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in a child with obesityFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in a child with obesityFrom the collection of Dr Jennifer Miller [Citation ends].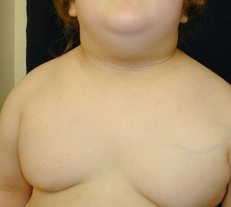 [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the folds of the neckFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the folds of the neckFrom the collection of Dr Jennifer Miller [Citation ends]. [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the axillaFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the axillaFrom the collection of Dr Jennifer Miller [Citation ends].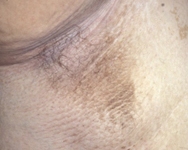
Not specific for type 2 diabetes mellitus, and can also be seen in children with obesity and type 1 diabetes mellitus.
polyuria
Typically present in patients with a fasting plasma glucose >300 mg/dL (>16.7 mmol/L) and/or HbA1c >10% (>13.3 mmol/mol).
polydipsia
Typically present in patients with a fasting plasma glucose >300 mg/dL (>16.7 mmol/L) and/or HbA1c >10% (>13.3 mmol/mol).
uncommon
nocturia
Due to glucose-induced diuresis.
Other diagnostic factors
common
hypertension
Frequently present at the time of diagnosis.
yeast infections
Most commonly in vaginal and penile areas, or in between skin folds.
skin infections
Cellulitis or abscesses.
urinary tract infections
Cystitis or pyelonephritis.
fatigue
Due to elevated glucose and/or comorbidities.
blurred vision
Due to elevated glucose and/or comorbidities.
uncommon
weight loss
Typically, little or no weight loss, although may be present if marked hyperglycemia is present.
Risk factors
strong
obesity
Children with obesity have hyperinsulinism, and they have approximately 40% lower insulin-stimulated glucose metabolism compared with children without obesity.[29][30][31]
Visceral fat is more metabolically active than subcutaneous fat and produces adipokines that cause insulin resistance. The amount of visceral fat in adolescents with obesity directly correlates with basal and glucose-stimulated insulin levels and inversely with insulin sensitivity.[42]
genetic predisposition/family history
A 3.5 times greater risk in siblings of affected individuals as compared with the general population.
About 80% to 100% concordance in monozygotic twins.[40]
More than 20 loci have been associated with type 2 diabetes mellitus (T2DM) in adults, the most important being NIDDM1, described among Mexican-American sibships in Starr County, Texas.[41]
African-American, Hispanic, American-Indian, and Asian or Pacific Islander
The majority of childhood-onset type 2 diabetes mellitus (T2DM) occurs in children from a high-risk racial/ethnic background.[9][10][11] Between 1990 and 1998, the number of American-Indian and Alaskan native children diagnosed with T2DM increased by 71%.[10] Although the risk groups can vary from country to country, the most at-risk group globally are Asian Indians.[12] As compared with white children, those of Asian Indian ancestry manifest adiposity, insulin resistance, and metabolic perturbations of obesity earlier in life, and have a tendency toward central adiposity even with a similar BMI.[13] One third of Mexican-American children and youth with diabetes in Southern California, and over two-thirds of those in South Texas, have T2DM.[14][15] Ethnic differences in background insulin sensitivity are also indicated by studies from Cincinnati, Arkansas, and Texas, where African-Americans account for 70% to 75% of pediatric T2DM.[16][17] Greater fasting and stimulated insulin responses to oral glucose, and less lipolysis, is seen in African-American prepubertal and pubertal children compared with European-Americans, adjusted for weight, age, and pubertal stage.[47]
puberty
Puberty is associated with relative insulin resistance, reflected by a two- to threefold increase in the peak insulin response to oral or intravenous glucose and a 30% lower insulin-mediated glucose disposal.[30] Puberty may also precipitate beta cell failure in the presence of preexisting insulin resistance.
female sex
In young-onset type 2 diabetes, females are affected more than males.[9]
weak
small for gestational age
In-utero programming as a result of prenatal growth restraint and limited nutrients in utero limits beta cell capacity and induces insulin resistance in peripheral tissues.[35]
rapid growth in infancy
diabetic in-utero environment
Children exposed to a diabetic intrauterine environment have a 3.7 times increased risk as compared with siblings born before the mother became diabetic.[34]
bottle feeding
Breast-feeding reduces the odds ratio for childhood obesity by approximately 20% as compared with formula feeding.[39] Formula feeding is more likely to be associated with overfeeding. Breast-feeding provides a more appropriate caloric intake at a critical stage in development.
high protein intake in infancy
polycystic ovaries
Associated with insulin resistance and hyperinsulinism, which predisposes to type 2 diabetes mellitus.
intramyocellular lipid content
In-vivo and in-vitro data suggest that elevated ceramide in skeletal muscle impairs insulin action, thus decreasing glucose uptake within the muscle.[44]
fat deposition in the liver
Elevations in alanine aminotransferase are associated with a decline in hepatic insulin sensitivity and the development of type 2 diabetes mellitus.[45]
Use of this content is subject to our disclaimer