Approach
A detailed history should be taken, with specific attention to gait abnormalities, urinary symptoms, and memory impairment. A thorough evaluation of the patient's gait is essential. Laboratory tests are generally unhelpful; however, neuroimaging is essential to rule out differential diagnoses. A diagnosis of NPH should be considered in any patient presenting with a gait apraxia (i.e., inability to walk in standing position, while able to make walking movements while lying). While initial investigation can be undertaken by a neurologist or neurosurgeon, more difficult cases that require prolonged external lumbar drainage or measurement of cerebrospinal fluid (CSF) impedance may require referral to a tertiary specialist center.
History
The major risk factors for NPH are age >65 years and the presence of vascular disease.[14][15][16]
Gait disturbances, such as difficulty or slowness in walking and loss of balance, are a key diagnostic factor and usually antedate other symptoms.[18]
Gait abnormalities may coexist with urinary frequency, urgency, or urge incontinence, or with cognitive impairment, including mental slowing (i.e., increased response latency, attention difficulties, impairment of abstract thinking and insight) and memory impairment (i.e., impaired recall, especially of recent events). These symptoms are generally insidious in onset, over months or years. Fecal incontinence is a feature of advanced disease and is rare.
Physical examination
A thorough evaluation of the patient's gait is essential. The cardinal feature of NPH is levodopa-unresponsive gait apraxia, characterized by:
A slow, cautious gait
Gait initiation failure
Unsteadiness (i.e., increased truncal sway while walking, retropulsion, widened standing base, impaired walking balance on tandem gait testing, impaired turning with 3 or more steps to turn 180 degrees)
Reduced stride length
Shuffling gait
Falls
Freezing.
Less common features include:
Festination (i.e., involuntary quickening of gait)
Tendency to get stuck in doorways
Extrapyramidal (predominantly resting) tremor
Stooped posture.
These signs are generally symmetric and predominantly affect the lower half of the body.
Neuroimaging
An MRI or a CT scan of the head (without contrast) should be ordered initially in all patients.[18] A CT scan provides sufficient information in the majority of cases, demonstrating the size and shape of the ventricles. However, an MRI scan has the benefit of avoiding exposure to ionizing radiation.
Neuroimaging is not diagnostic by itself, but is necessary to exclude other pathology. The scan may be normal, or may show mild to moderate dilation of the ventricles and periventricular leukomalacia (i.e., damage to the white matter around the cerebral ventricles). There may be disproportionate central atrophy, resulting in larger ventricles with relative preservation of cortical sulci in the atrophic process.[19] An obstructive lesion excludes a diagnosis of NPH.
Advanced vascular disease suggests a worse prognosis. Turbulent flow of CSF in the aqueduct demonstrated on MRI may be associated with shunt responsiveness.[Figure caption and citation for the preceding image starts]: Normal CT scan in patient with levodopa-unresponsive gait apraxia, emphasizing that the condition is not necessarily a radiologic diagnosisPersonal collection of Richard Adam Grünewald [Citation ends].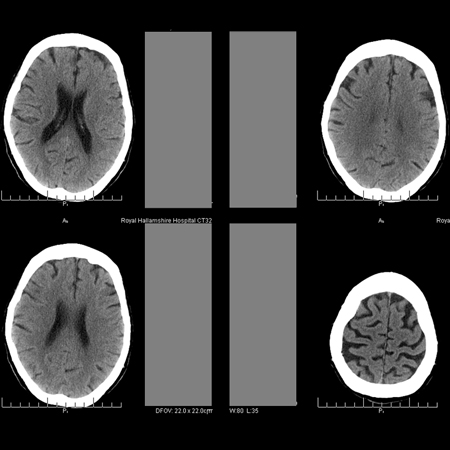 [Figure caption and citation for the preceding image starts]: MRI fluid attenuation inversion recovery (FLAIR) sequence showing periventricular high signal (leukoaraiosis) and central and peripheral brain atrophyPersonal collection of Richard Adam Grünewald [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI fluid attenuation inversion recovery (FLAIR) sequence showing periventricular high signal (leukoaraiosis) and central and peripheral brain atrophyPersonal collection of Richard Adam Grünewald [Citation ends].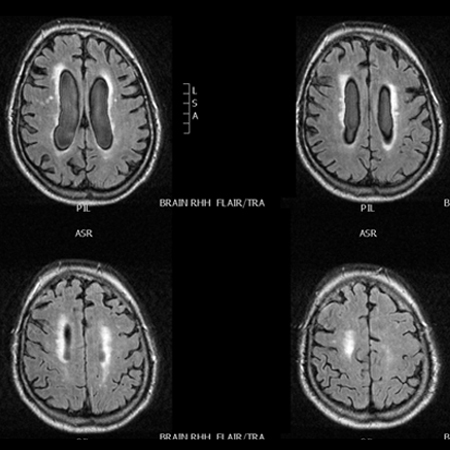
Levodopa challenge
A therapeutic trial of levodopa should be given to all patients where Parkinson disease is suspected.[20] The patient is then asked to note whether there is any subjective improvement in gait symptoms. If symptoms are responsive, a diagnosis of NPH can be ruled out.
If there is any doubt about the response, a formal challenge test should be considered, where motor function is assessed quantitatively, rather than subjectively, before and after levodopa is given. A timed walk is recorded on video, followed by administration of levodopa. The timed walk is then repeated after 1 to 3 hours and the result compared with the initial timed walk. A positive response to the drug is represented by a clinically useful improvement in walking speed (approximately >20%). The patient should also be asked to report any subjective improvement. Occasionally, more delayed improvement is seen, but symptoms usually gradually recur over the days following the test. Levodopa challenge should only be undertaken after an overnight withdrawal of all dopaminergic drugs.
Additional testing
Some institutions recommend timing the patient getting out of a chair and a 180-degree turn, if the patient's gait apraxia allows, before and after levodopa challenge. Levodopa challenge should only be undertaken after an overnight withdrawal of all dopaminergic drugs.
Other investigations
Lumbar puncture:
Should be considered in any patient with levodopa-unresponsive gait apraxia, unless:
a corrective surgical procedure is considered unsuitable, after an assessment of the benefits and risk of surgery
a definitive alternative diagnosis is established.
Reveals normal (or not significantly elevated) CSF pressure.[18]
Lumbar puncture with large-volume CSF tap:
Should be considered in any patient with levodopa-unresponsive gait apraxia.
Involves the removal of 30 to 60 mL of CSF via lumbar puncture.
Supports a diagnosis of NPH, and indicates an increased likelihood of response to a CSF diversion procedure, if there is an improvement in gait symptoms, shown by a recorded, timed walk (and other tests, as described above, if appropriate) before and 1 to 3 hours after the procedure.
Although the sensitivity of the test is uncertain, it is thought to be of high specificity.
Considered diagnostic, prognostic, and therapeutic. Test is therapeutic as it improves cerebral blood flow temporarily, possibly by reducing the turgor of the subarachnoid space and relieving obstruction to CSF flow at the aqueduct.[21]
Prolonged external lumbar drainage:
Usually reserved for patients with equivocal results after large-volume CSF tap, or if there is a high index of suspicion of diagnosis but no response to large-volume CSF tap.
Involves placing a catheter in the lumbar CSF space for 2 to 4 days and removing 150 to 250 mL of CSF per day.
Supports a diagnosis of NPH if there is an improvement in gait symptoms, shown by a recorded, timed walk (and other tests, as described above, if appropriate) before and 1 to 3 hours after the procedure.
May have a higher sensitivity than a single, large-volume CSF tap, but the evidence for this procedure is sparse.
Available in specialized centers only.
CSF infusion procedure:
Usually reserved for patients with equivocal results after large-volume CSF tap, or if there is a high index of suspicion of diagnosis but no response to large-volume CSF tap.
Measurement of the impedance to flow of the CSF pathway can be performed in several ways; elevated outflow resistance predicts which patients will respond most favorably to shunting.[22]
In the Katzman test, a pump infuses saline through a needle in the lumbar subarachnoid space; the impedance is calculated as the difference in the final steady state pressure reached and the initial pressure, divided by the infusion rate.
Alternative methods involve bolus injections of volumes of saline into the subarachnoid space, or rely on recording the time pressure curve during continuous saline infusion.
The results of these tests are variable and center-specific, but the technique of CSF impedance measurement has been assessed as potentially valuable.[23]
Available in specialized centers only.
Continuous intracranial pressure measurement:
Has been used in the assessment of NPH, but there are no data to support its routine use in diagnosis.
One prospective cohort study (n=131) of patients undergoing surgery for NPH reported that 93% of patients with increased intracranial pressure (ICP) pulsatility (ICP wave amplitude >4 mmHg on average and >5 mmHg in >10% of recording time) responded to shunt surgery, whereas 10% of patients without increased ICP pulsatility responded to shunt surgery.[24]
Another prospective study found that high ICP pulse amplitude had a sensitivity of 84.4%, specificity of 88%, positive predictive value of 94.7%, and negative predictive value of 61.8% for predicting shunt responders.[25]
There is a small risk of superficial wound infection and ventriculitis associated with this procedure.
Use of this content is subject to our disclaimer