Etiology
Causes of CP are multifactorial, although in 30% of patients there is no known risk factor or identifiable etiology. Risk factors for CP may be divided into prenatal, perinatal, and postnatal.[22][23][24] Cerebral Palsy Foundation fact sheet: risk factors Opens in new window
Prenatal factors
These include prematurity; multiple births; maternal illnesses such as thyroid disease, iodine deficiency, TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) infections, thrombotic disorders including factor V Leiden mutations, and chorioamnionitis; teratogen exposure; genetic and metabolic disorders; and fetal brain malformations.[25][26][27] Low income levels were associated with a twofold increase in CP in one study.[17]
Approximately 70% to 80% of cases of CP are thought to be due to prenatal causes.[22] The primary risk factors are prematurity, multiple births, and maternal infections such as chorioamnionitis.
Approximately 35% of children born at less than 26 weeks' gestation develop CP.[28] Multiple births are at an increased risk of CP; in one UK study, the reported prevalence of CP per 1000 live births was 2.3 for singletons, 12.6 for twins, and 44.8 for triplets.[16] Infants born at 22 and 23 weeks' gestational age have a substantially higher likelihood of death or neurodevelopmental impairment compared with those born at 24 weeks' gestational age or greater.[29]
In one case-control study of term singletons, birth defects in combination with growth restriction contributed substantially to the risk for CP.[30][31] Birth defects were recognized in 5.5% of neonates in the control group compared with more than half of neonates with CP (without hypoxic-ischemic encephalopathy).[30] While birth defects are more commonly detected in preterm infants in the general population, there is an increase in major birth defects (by a factor of 9) in full-term children with CP compared with preterm babies with CP.[32]
Hemiplegia may be due to focal lesions in utero, or to neonatal vascular accidents, including emboli from thrombosis in the placenta. Mothers who have factor V Leiden may be predisposed to this etiology.[25][26]
Perinatal factors
These include birth asphyxia due to instrumental delivery, nonvertex presentation, birth trauma, placental abruption, rupture of the uterus or prolonged/obstructed labor, and postmaturity. However, less than 10% of cases of CP are believed to be related to birth asphyxia, and use of electronic fetal monitoring has not been shown to be a factor in preventing CP.[33][34]
Postnatal factors
These include hyperbilirubinemia, neonatal sepsis, respiratory distress, early-onset meningitis, intraventricular hemorrhage, and head injuries prior to 3 years (including those due to child abuse and shaken baby syndrome).
Around 25% of infants who survive neonatal seizures have CP.[35]
Pathophysiology
The pathophysiology of CP varies depending on the etiology, which includes overt structural lesions of the brain or less visible lesions that result from prenatal, neonatal, and postnatal events. Other factors such as toxins, infections, multiple births, and maternal health also exert an influence.[22]
Postnatal brain magnetic resonance imaging scans show abnormalities in up to 80% of established cases of CP.[36][37]
Severe compromise in oxygen and/or cerebral perfusion following birth trauma leads to hypoxic-ischemic encephalopathy. A similar neonatal encephalopathy can occur with inflammatory conditions such as maternal fever and infection.[38][39] Between 26 and 34 weeks' gestation, selective vulnerability of the periventricular white matter (e.g., to periventricular leukomalacia or periventricular hemorrhage) occurs involving the internal capsule (a white matter structure composed of bundles of myelinated fibers that carry information to and from the cerebral cortex). Fetal insults at this time may result in spastic diplegia. These white matter changes are found in 71.3% of children with spastic diplegia, 34.1% with spastic hemiplegia, and 35.1% with spastic quadriplegia, despite 25% of these children being term births.[36][Figure caption and citation for the preceding image starts]: The lateral corticospinal tract (shown in red), responsible for voluntary motor controlTeachMeAnatomy (https://teachmeanatomy.info/neuroanatomy/pathways/descending-tracts-motor/); used with permission [Citation ends].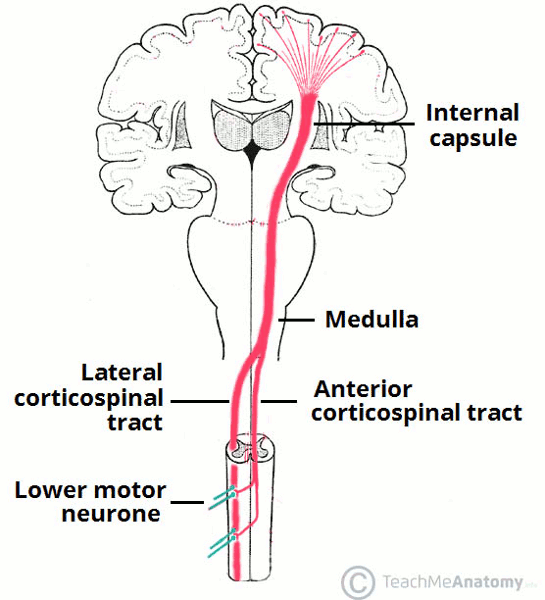
In spastic CP, impulses from the motor cortex (precentral gyrus) are compromised along their path via the internal capsule to the spinal motor neurons and eventually to the site of action, the skeletal muscles. The corticospinal (pyramidal) tracts initiate and carry impulses necessary for voluntary movement. Damage to the primary motor cortex or the corticospinal tract impairs voluntary movement and fine motor control. Spasticity appears to be a result of damage or abnormal input to the vestibular and reticular nuclei or their tracts, which results in loss of inhibitory influences to the spinal motor neuron pools. Damage to the reticulospinal system increases tone; damage to the vestibulospinal tract, which modulates antigravity skeletal activity and balance reflexes, results in an increased extensor tone.[40]
Lesions of the basal ganglia, commonly associated with parkinsonian-like syndromes, do not result in spasticity but are involved in the dyskinetic forms of CP. Unique metabolic demands of the basal ganglia in the fetus at 38 to 40 weeks can result in dystonia or other movement disorders in the event of damage during this period.[41] Severe hyperbilirubinemia, now largely preventable, leads to damage to basal ganglia due to deposition of bilirubin byproducts, causing dyskinesia. Ataxia and hypotonia are associated with damage to the cerebellum or cerebellar pathways.[40]
Classification
CP is currently classified according to motor impairment, anatomic distribution, and functional level.[1]
Movement disorder classification[2]
Spastic
Most common subgroup, characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks and clonus resulting from hyperexcitability of the stretch reflex.[3] Patients tend toward hip adduction, knee flexion, and ankle plantar flexion (equinus). The upper extremities, when involved, tend toward forearm pronation, wrist flexion, elbow flexion, and thumb-in-palm deformities. Based on topography, spastic CP can be further classified as:[4]
Monoplegia: single limb involvement.
Hemiplegia: involvement of an ipsilateral upper and lower extremity. Hemiplegia is also described as unilateral CP.
Diplegia: predominant involvement of both lower extremities, significantly greater than the upper extremities. Diplegia is also described as bilateral CP and further classified by gross motor function classification system (GMFCS) level, most frequently levels I-III.[5]
Quadriplegia: involvement of all limbs and the trunk. It is also called "total body involvement" in patients with involvement of muscles of the neck, phonation, and swallowing mechanisms. The term bilateral hemiplegia may also be used when one side has a significantly different tone compared with the other. Quadriplegia is also described as bilateral CP and further classified by GMFCS level, most frequently levels IV-V.[5]
Dyskinetic
Involuntary, recurring, and occasionally stereotyped movements with a varying muscle tone. Subgroups include:
Dystonia: characterized by involuntary, sustained contractions resulting in twisting and abnormal postures.
Chorea: rapid, involuntary, jerky, and fragmented motions; tone is usually decreased but fluctuating.
Athetosis: slower, constantly changing, writhing, or contorting movements.
Ataxic
Loss of muscular coordination with abnormal force and rhythm, and impairment of accuracy. Commonly presents with gait and trunk ataxia, poor balance, past pointing, terminal intention tremor, scanning speech, nystagmus and other abnormal eye movements, and hypotonia.
Mixed
Most patients have a predominant motion disorder but when that cannot be determined, patients are classified as having a mixed-type CP.
Other classifications
CP can be classified on its anatomic distribution as unilateral (hemiplegia) or bilateral (diplegia and quadriplegia). Bilateral cases, upon close examination, may not be perfectly symmetrical.
CP can also be simply subdivided as ambulatory or nonambulatory.
In clinical practice and research, CP is classified by functional impairment/independence in the areas of gross motor, fine motor, and communication.[6]
Differentiating patients into subtypes is helpful as it leads to group-specific treatment recommendations. The International Classification of Diseases (ICD) codes for various subtypes of CP and other paralytic syndromes are available from the World Health Organization. WHO: ICD codes - cerebral palsy and other paralytic syndromes Opens in new window
Gross motor function classification system (GMFCS)[7][8]
This classification system, for children ages 2 to 18 years, is a measure of mobility function. The focus of the GMFCS is on determining which level best represents the child’s or youth’s present abilities and limitations in gross motor function.
Level I. Walks unimpeded without assistive devices; has impairments in advanced gross motor skills.
Level II. Walks mostly without assistive devices; uses railings for stairs; has difficulty walking outdoors on uneven terrain or in the community.
Level III. Walks with assistive devices such as crutches or a walker; may use wheelchair for long distances.
Level IV. Limited self-mobility; transported using wheelchair or uses power chair as primary mobility; may walk for short distances with assistance.
Level V. Total dependence on others for wheelchair mobility; if using power chair, requires extensive adaptation.
Updated classification schemes with clarifications and revisions and classifications for younger children can be obtained from the CanChild Centre. CanChild: Gross Motor Function Classification System - Expanded & Revised (GMFCS - E&R) Opens in new window GMFCS handouts for different age-groups are also available.
Manual ability classification system (MACS)[9] Manual Ability Classification System Opens in new window
This system classifies how children with CP ages 4 to 18 years use their hands to handle objects in their daily life. It does not grade each hand individually, but rather gives a single score based on how the child usually and typically manipulates objects at home, school, and in community settings. It can be broadly summarized as follows.
Level I: Handles objects easily and successfully.
Level II: Handles most objects but with somewhat reduced quality and/or speed.
Level III: Handles objects with difficulty; needs help to prepare and/or modify activities.
Level IV: Handles a limited selection of easily managed objects in adapted situations.
Level V: Does not handle objects and has severely limited ability to perform even simple actions.
Communication functional classification system (CFCS)[10] Communication Function Classification System Opens in new window
This system classifies the everyday communication performance of individuals with a disability over the age of 4 years. It describes the ability of the person to both send and receive information. While the method of communication is noted, the use of an augmentative or alternative communication system (e.g., communication device or word board) does not affect the level given. The levels can be broadly summarized as follows.
Level I: Effective sender and receiver with unfamiliar and familiar partners.
Level II: Effective but slower-paced sender and/or receiver with unfamiliar and/or familiar partners.
Level III: Effective sender and receiver with familiar partners.
Level IV: Inconsistent sender and/or receiver with familiar partners.
Level V: Seldom effective sender and receiver even with familiar partners.
Use of this content is subject to our disclaimer