Approach
For patients with neurogenic thoracic outlet syndrome (TOS), initial treatment is conservative and focuses on physical therapy. Surgical management of neurogenic TOS is indicated in individuals who have disabling symptoms and have failed conservative treatment, or in those who show physical signs of nerve damage. For patients with venous or arterial TOS, prompt thrombolysis and/or surgical intervention is often required. Patients with symptomatic intermittent venous obstruction (McCleery syndrome) or patients with chronic subclavian vein thrombosis can be scheduled for semielective surgery. Comparison of different treatments for TOS is complicated by use of different diagnostic criteria and a lack of high-quality randomized studies.[32]
Neurogenic TOS (NTOS)
Treatment is primarily conservative and focuses on physical therapy.[139][140] Surgical intervention is reserved for patients with substantial functional disability and an insufficient response to appropriate conservative treatment. One randomized, prospective, clinical trial demonstrated significantly better outcomes for early surgery in patients whose symptoms are refractory to physical therapy, compared with continuing physical therapy.[141] Early surgical treatment is also recommended for those with electrodiagnostic abnormalities, motor deficits, and muscle atrophy at initial presentation (i.e., Gilliatt-Sumner hand).[140] The preferred surgical approach depends largely on the training and experience of the surgeon.
Patients often present with sensory symptoms and no objective evidence of nerve injury on exam or diagnostic studies such as electrodiagnostic testing. Most patients are initially treated conservatively with core strengthening therapies that help to improve posture and realign musculoskeletal structures. Physical therapy is also used to enhance the space between the clavicle and first rib, improve posture, strengthen the shoulder girdle, and loosen the neck muscles.[142] This is accomplished by pectoralis stretching, strengthening the muscles between the shoulder blades, good posture advice, and active neck exercises (including chin tuck, flexion, rotation, lateral bending, and circumduction).[139] Ergonomics are also a key factor in rehabilitation and the ability of the patient to return to work. Other conservative therapies include rest, appropriate work restrictions, and pharmacotherapy for pain relief.
Pharmacotherapy should be limited to oral analgesics such as nonsteroidal anti-inflammatory drugs and muscle relaxants. Opioids should be avoided.
Local anesthetic muscle injections are largely used for aiding diagnosis and prognosis, but do not provide sustainable pain control. Botulinum toxin injections into the scalene and pectoralis minor muscles may be used; however, this treatment has not shown long-term efficacy.[32][143][144][145][146] Neurogenic TOS is not an approved indication for botulinum toxin treatment and there is no clear evidence that treatment with botulinum toxin affects the success of physical therapy treatment for neurogenic TOS. One controlled study in patients with neurogenic TOS found no difference in outcomes (or the need for surgery) after muscle injections with botulinum toxin vs saline alone.[145]
The usual indications for surgery are insufficient improvement after appropriate conservative therapy in a patient with a sound clinical diagnosis (no other condition thought to explain symptoms) and substantial disability.[140] Some surgeons prefer to limit operation to those patients that have had a positive response to a scalene and pectoralis minor muscle block, but this is not a requirement for successful surgical treatment.[118][136] Although isolated pectoralis minor tenotomy is considered for findings confined to this muscle, only about 10% of patients have these confined findings. For those where tenotomy is insufficient, due to additional involvement at the scalene triangle, approximately 75% to 85% will require further surgical interventions such as scalenectomy and first rib resection.[51] Patients with clinical findings confined to the pectoralis minor space may be considered for isolated pectoralis minor tenotomy.[50][51] The cause of brachial plexus compression in the vast majority of patients with a Gilliatt-Sumner hand will involve a bony structure, such as a cervical rib. Given the advanced nature of the neurologic deficit in these patients, early surgical intervention is the preferred approach as most will not respond to conservative management.[31][140] A supraclavicular or transaxillary approach can be used in these patients.
The commonly performed surgical procedures for the treatment of neurogenic TOS typically include at least one of the following components: 1) removal of anomalous structures (e.g., cervical rib, muscle anomalies, fibrous bands); 2) removal of the first rib; 3) partial or complete removal or division of the anterior scalene and middle scalene muscles; 4) external neurolysis of the supraclavicular brachial plexus nerves; and 5) release of the pectoralis minor muscle. The most commonly used surgical approaches are the supraclavicular and transaxillary approaches. Additionally, robotic-assisted transthoracic approaches have been introduced. While the transthoracic first rib resection shares conceptual similarities with the transaxillary approach, including the potential benefits and risks, research does not indicate clear advantages of transthoracic methods over the more traditional approaches for thoracic outlet decompression.[147][148][149] The approach used is generally based on surgeon and patient preference.[1]
The advantage of the supraclavicular approach is the relative ease of exposure of the scalene muscles, subclavian artery, and brachial plexus, phrenic, and long thoracic nerves. In addition to first rib resection, this permits a complete scalenectomy and brachial plexus neurolysis. Disadvantages include the need to manipulate the brachial plexus nerves and subclavian vessels, as well as potential injury to the exposed neurovascular structures. Hospital stay is typically several days longer than that for alternative procedures. The main advantages of the transaxillary approach are a hidden incision, the diminished need to retract the brachial plexus and major vessels, ease of exposure of the lower trunk of the brachial plexus (C8 and T1 nerve roots), and shorter hospital stay (typically 1-2 days). A disadvantage is more limited exposure that prevents complete scalenectomy or brachial plexus neurolysis (the C5, C6, and C7 nerve roots cannot be exposed), and potential traction-related dysfunction of the intercostobrachial nerve. There is no evidence to indicate that one approach has consistently or significantly better results than the other, but long-term recurrence rates may be higher following transaxillary first rib resection than after supraclavicular decompression.[150][151][152][153][154]
Pectoralis minor tenotomy can be considered in cases of neurogenic TOS where there is evidence from clinical exam for brachial plexus nerve compression in the subcoracoid space. Some consider this a frequent and important component of primary surgical treatment, whereas others reserve this for patients experiencing later recurrence of symptoms after initial surgical intervention. This procedure can be performed by making a small vertical incision in the deltopectoral groove or axilla, elevating the pectoralis major muscle to expose the pectoralis minor muscle and coracoid process, and division of the pectoralis minor tendon from its attachment.[51]
Surgical complications in general include injury to the neural structures (i.e., brachial plexus, intercostal nerve, phrenic nerve, long thoracic nerve), bleeding, infection, pneumothorax, hemothorax, localized lymph leak or chylothorax, and incomplete nerve release. Pain control with oral and/or intravenous analgesics is appropriate in the immediate postoperative period, with the selection of the specific analgesic agent depending on physician preference. Oral analgesics often need to be continued after the immediate postoperative period. Physical therapy is paramount for postoperative rehabilitation, focusing on posture modifications, stretching and muscle relaxation, shoulder girdle strengthening, and ergonomics. Rest and work restrictions are also recommended.
[Figure caption and citation for the preceding image starts]: Transaxillary first rib resection and scalenectomy. (A): Patient position. (B): Anatomical features of the thoracic outlet as viewed from the lateral approach. (C): Exposure of the first rib high in the axilla, with the associated muscles and subclavian vessels visualized. (D): Dissection along the periosteum of the first rib. (E): Middle scalene and subclavius muscles are divided and the anterior scalene is encircled prior to its division. (F): Division of the posterior first rib. ASM: anterior scalene muscle; BP: brachial plexus; C8-T1: C8 and T1 cervical nerve roots; MSM: middle scalene muscle; SCA: subclavian artery; SCV: subclavian vein; SubCM: subclavius muscleArnaoutakis GJ, et al. Atlas of Vascular Surgery and Endovascular Therapy: Anatomy and Technique. Chaikof EL, Cambria RP, editors. Chapter 16. Transaxillary rib resection for thoracic outlet syndrome. Elsevier Saunders: Philadelphia; 2014. Pages 193-203; used with permission. [Citation ends].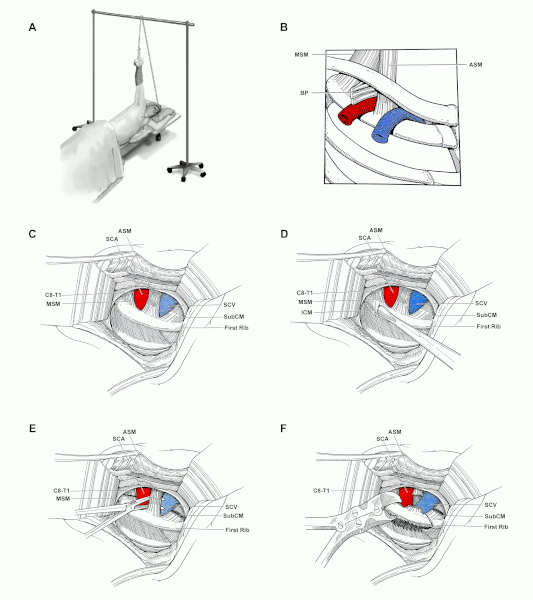
Venous TOS
Immediate venography and catheter-directed intervention is strongly advised within the first 2 weeks of diagnosis of subclavian vein thrombosis but may still be effective if performed up to 6-8 weeks after the onset of arm swelling symptoms. However, the success rate of these treatments declines significantly after 6-8 weeks due to progressive fibrosis in the affected vein, highlighting the importance of timely intervention.Catheter-directed treatment for venous thrombosis should be conducted within 2-3 weeks of symptoms and has a low rate of success after 6-8 weeks, due to progressive fibrosis in the affected vein.[113] If acute thrombosis is present, catheter-directed therapy is recommended with thrombolysis, suction thrombectomy, and/or balloon angioplasty. Placement of stents in the subclavian vein is contraindicated in the absence of surgical decompression.[155] Venography is performed through an antecubital, basilic, or brachial vein catheter and thrombolytic treatment and/or suction thrombectomy are initiated. The thrombolytic agent chosen and method of clot disruption is determined by the treating physician. Surgical treatment may be performed immediately after catheter-directed therapy, within days during the same hospital stay, or after an interval of 4-6 weeks with the patient remaining on anticoagulation treatment.[156][157][158][159]
The surgical approach selected should ensure decompression at the costoclavicular space, with resection of the anterior scalene muscle, the subclavius muscle and costoclavicular ligament, and the anteromedial first rib. This can be accomplished by transaxillary, robotic-assisted transthoracic, infraclavicular, or paraclavicular approaches (the isolated supraclavicular approach does not facilitate adequate resection of the anteromedial first rib). The approach used is generally based on surgeon experience and preference.[79][91][160][161][162][163][164][165]
Intraoperative venography can help define the degree of vein obstruction present at the time of surgery and immediately after decompression, and it can help guide vein repair if needed. Intravascular ultrasound may be another option for assessing the subclavian vein. In some cases, intraoperative balloon angioplasty may be considered for residual vein stenosis. Direct axillary-subclavian vein reconstruction can be accomplished at the time of surgery when the infraclavicular or paraclavicular approaches are used, with either patch angioplasty or bypass grafting. With the transaxillary approach, venography is sometimes deferred for several weeks after surgery and endovascular approaches (e.g., balloon angioplasty) are used to treat any residual vein stenosis; however, with the availability of a hybrid operating suite this can be easily combined with the primary decompression procedure. Placement of subclavian vein stents following thoracic outlet decompression has shown promising results.[166][167][168][169] Complications of surgery include injury to neural structures (i.e., brachial plexus, intercostobrachial nerve, phrenic nerve), bleeding, infection, pneumothorax, pleural effusion, chylothorax, and postoperative subclavian vein thrombosis.
Many patients with longstanding axillary-subclavian vein thrombosis will eventually exhibit minimal or no symptoms, with collateral venous pathways sufficient to prevent arm swelling or congestive symptoms. Surgical treatment to decompress the thoracic outlet can improve collateral pathways even if the subclavian vein cannot be reopened or a bypass performed.[91][156][170][171][172]
Patients with compromised hemodialysis access due to venous TOS are at considerably higher risk for surgery, so initial treatment with endovascular approaches is preferred. If there are repeated occurrences of access thrombosis or persistent arm swelling, thoracic outlet decompression is considered. This may be performed by an infraclavicular approach with limited anterior first rib resection, or by resection of the medial clavicle as an alternative.[81][82][83][84][85][86][87]
Patients are typically discharged from hospital on anticoagulation following surgery, for periods of 1-3 months. Anticoagulation is most frequently accomplished with a direct oral anticoagulant (DOAC). Other options include warfarin or a low-molecular-weight heparin (e.g., enoxaparin). Antiplatelet agents (e.g., aspirin, clopidogrel) may be used in some cases. Physical therapy is paramount during postoperative rehabilitation, focusing on posture modifications, stretching and muscle relaxation, shoulder girdle strengthening, and ergonomics. Pain control with oral and/or intravenous analgesics is appropriate in the immediate postoperative period, with the selection of the specific analgesic agent depending on physician preference. Oral analgesics often need to be continued after the immediate postoperative period. Rest and work restrictions are also recommended.
Arterial TOS
Immediate surgical intervention is required if there is concern about acute limb ischemia. Emergency surgical treatment may include initial exploration at the brachial artery level for thromboembolectomy, with catheter-directed thrombolysis as another treatment option.[56] Intraoperative adjuncts include systemic anticoagulation and possible infusion of thrombolytic agents into the distal artery branches. Adjunctive forearm fasciotomy may also be needed in cases of prolonged acute ischemia. Once acute limb ischemia is resolved, thoracic outlet decompression should be undertaken either at the same setting or within days to weeks, while the patient is maintained on anticoagulation treatment.
For patients with a cervical rib, decompression for arterial TOS can be accomplished by either transaxillary or supraclavicular approaches with resection of the cervical rib and first rib, resection of the anterior and middle scalene muscles, and removal of congenital bands and accessory muscles. The subclavian artery must be assessed for the presence of aneurysmal dilatation. Intraoperative assessment of the subclavian artery can include direct observation, intraoperative arteriography, or intravascular ultrasound. The presence of aneurysmal dilatation (diameter greater than twice normal), mural thrombus, or thromboembolism are all indications for arterial repair. Milder degrees of poststenotic dilatation without mural thrombus may be observed after decompression without direct arterial repair. When needed, subclavian artery reconstruction should be performed with a supraclavicular approach, with or without an infraclavicular incision for exposure of the distal artery. The aneurysmal segment of subclavian artery is excised with interposition bypass graft repair. Conduits may include prosthetic grafts, autologous reversed saphenous vein, or cryopreserved femoral artery or vein allografts. Endovascular placement of stents in the subclavian artery is an emerging alternative option for vascular reconstruction.[54][55]
Postoperative evaluation of arterial patency is essential. Perfusion of the limb and pulses should be routinely tested clinically. In cases where clinical assessment is in question, assessment of arterial patency with arteriography or CT angiography may be necessary. Arteriography also allows for diagnosis and immediate treatment of complications, such as dissection, thrombosis, or pseudoaneurysm. Complications include injury to neural structures (i.e., brachial plexus, intercostobrachial nerve, phrenic nerve), bleeding, infection, pneumothorax, pleural effusion, chylothorax, and postoperative arterial thrombosis.
Postoperative anticoagulation is necessary after surgical treatment with thromboembolectomy, direct arterial repair, if residual ischemia is present during postoperative evaluation, or if the patient is deemed to have a hypercoagulable state. Anticoagulation is most frequently accomplished with a DOAC. Other options include warfarin or a low-molecular-weight heparin (e.g., enoxaparin). Antiplatelet agents (e.g., aspirin, clopidogrel) may be used in some cases.
Physical therapy is paramount during postoperative rehabilitation, focusing on posture modifications, stretching and muscle relaxation, shoulder girdle strengthening, and ergonomics. Pain control with oral and/or intravenous analgesics is appropriate in the immediate postoperative period, with the selection of the specific analgesic agent depending on physician preference. Oral analgesics often need to be continued after the immediate postoperative period. Rest and work restrictions are also recommended.
Combined forms of TOS
Immediate surgical intervention is required for both the vascular and neurogenic components of combined TOS. Techniques include catheter-directed thrombolysis and surgical decompression of the thoracic outlet. The method selected depends on the pathology and type of thoracic outlet syndrome and surgeon preference.
Transaxillary approach:[173][174]
General anesthesia is used and may be complemented by placement of regional anesthesia blocks prior to or after operation.[175][176][177][178][179][180][181]
The patient is positioned on their side with the affected arm raised and held with a self-retaining retraction device. A transverse incision is made along the axilla at the lower edge of the axillary hairline, from the edge of the latissimus dorsi muscle to the edge of the pectoralis major muscle. The exposure is carried through subcutaneous and fascial tissues to the chest wall, avoiding division or undue retraction on the intercostobrachial nerve. The exposure is advanced superiorly along the chest wall, underneath the axillary contents, to the level of the first rib. The neurovascular structures are identified and protected (subclavian vein, subclavian artery, and the lower trunk of the brachial plexus). The anterior scalene muscle is circumferentially exposed several centimeters above its attachment to the superior edge of the anterior first rib and divided with scissors. The attachment of the middle scalene muscle is divided from the upper surface of the posterior first rib. Under apnea with the lungs collapsed, the periosteum of the first rib is scored with a cautery and the intercostal muscle is divided along the outer (inferior) edge of the first rib. The attachments of the subclavius muscle and costoclavicular ligament are divided from the upper surface of the first rib, and the anterior first rib is divided at the costal cartilage junction. The lower trunk of the brachial plexus is exposed along with the C8 and T1 nerve roots at the level of the posterior first rib. The posterior first rib is divided, ideally at the costotransverse joint, and the first rib specimen is removed.
For neurogenic TOS, any apparent fibrous bands or scar tissue adjacent to the lower trunk of the brachial plexus are resected. In some cases, the lower trunk may be partially wrapped with an absorbable film barrier to help reduce postoperative scarring around the nerves.
For venous TOS, any fibrous tissue around the subclavian vein is resected and the vein is assessed for patency. A follow-up venogram is typically planned for several weeks after surgery, at which time any residual venous stenosis is addressed with endovascular techniques (e.g., balloon angioplasty); alternatively, a completion venogram can be performed at the time of primary operation and a low pressure balloon inflation can be used to assess any residual venous stenosis.
For arterial TOS (or neurogenic TOS) with a cervical rib, this can be removed prior to first rib resection by extending the exposure somewhat higher and dividing the posterior cervical rib at the costotransverse junction. The anterior aspect of the cervical rib is detached from any connection to the first rib, and the cervical rib is removed as a specimen. The subclavian artery is assessed, and if reconstruction is required, the patient is repositioned for an anterior (supraclavicular) approach. In some cases, a follow-up arteriogram is planned following surgery, with any arterial pathology to be addressed by endovascular approaches (e.g., balloon angioplasty and/or stent placement).
Upon completion of the procedure the pleura is inspected and if it has been opened, a small drain is placed into the pleural space and attached to suction. Alternatively, some surgeons will prefer to place a drain regardless of pleural entry. The incision is closed in layers.
Robotic video-assisted transthoracic approach:[147][148][149]
General anesthesia is used. Following placement of a double lumen endotracheal tube the patient is positioned in full lateral decubitus. Three 8-mm robotic ports and a 10-mm assistant port are placed. The 8-mm ports are generally placed in the fourth intercostal space anterior to the anterior axillary line, seventh intercostal space midaxillary line, and sixth intercostal space posterior to the scapular tip. The entire first rib is visualized without retraction of any structure and the first intercostal muscle is separated from the lateral aspect of the first rib. The mediastinal pleura is swept off the inferior aspect of the rib and the costal cartilage is divided with electrocautery. The posterior arm of the robot is de-docked, and a handheld bone drill is placed through the posterior incision to divide the rib at the level of the transverse process. The robot arm is re-docked, the rib is reflected medially, and the middle scalene, anterior scalene, and subclavius muscles are separated from their insertions on the first rib. The first rib is then removed through the assistant port incision. No retraction or manipulation of the brachial plexus, subclavian artery or vein, or phrenic nerve is required. Partial resection of the anterior scalene and middle scalene muscles is performed with robotic scissors. For neurogenic TOS, lower trunk brachial plexus neurolysis is performed sharply using a robotic scissors. For venous TOS, external venolysis of the subclavian vein is performed. Upon completion of the procedure, an intrapleural drain is placed, the lung is expanded, and the incisions are closed.
Supraclavicular approach:[182]
General anesthesia is used and may be complemented by placement of regional anesthesia blocks prior to or after operation.[175][176][177][178][179][180][181]
The patient is positioned supine with the arm resting along the side. A transverse incision is made above the clavicle, from the border of the trapezius muscle to the edge of the sternocleidomastoid muscle. The exposure is carried through the subcutaneous and platysma layers, with division or retraction of the supraclavicular cutaneous sensory nerves. The scalene fat pad is exposed and detected from its superior, medial, and inferior attachments, then rotated laterally, with division of the omohyoid muscle, and all the structures of the scalene triangle are identified. The insertion of the anterior scalene muscle is circumferentially dissected at its insertion on the first rib, protecting the brachial plexus and subclavian artery. The anterior scalene muscle is divided from the first rib and elevated, then dissected away from the underlying brachial plexus. The muscle is passed underneath the phrenic nerve and exposure is extended superiorly to the apex of the scalene triangle, where the origin of the anterior scalene is divided and the muscle is removed as a specimen. The brachial plexus is separated from the middle scalene muscle to exposure all three trunks, which are gently retracted medially. The long thoracic nerve is gently retracted laterally and the insertion of the middle scalene muscle on the posterolateral first rib is exposed. The middle scalene is divided from the first rib with a cautery, extending to the posterior first rib with exposure of the C8 and T1 nerve roots. The middle scalene muscle is divided with visualization and preservation of the long thoracic nerve and removed as a specimen. The posterior first rib is circumferentially exposed and divided at the level of the costotransverse joint. The intercostal muscle attachments to the rib are divided and the undersurface of the rib is mobilized. The anterior first rib is divided at the medial edge of the scalene tubercle and the rib is removed as a specimen. The subclavian artery and brachial plexus nerves are fully mobilized with resection of any adherent fibrous scar tissue and ligamentous bands. For neurogenic TOS, the brachial plexus may be loosely wrapped with an absorbable film barrier or a portion of the scalene fat pad to help reduce postoperative scarring around the nerves.
For arterial TOS (or neurogenic TOS) when a cervical rib is present, this can be removed prior to first rib resection. The cervical rib is encountered within the plane of the middle scalene muscle and the posterior aspect of the cervical rib is circumferentially exposed and divided at the level of the costotransverse joint. For a complete cervical rib, the anterior aspect of the cervical rib can be left attached to the first rib and removed as a single specimen. For a partial cervical rib, the anterior aspect of the rib terminates in a ligamentous band, which is divided to allow the cervical rib specimen to be removed prior to first rib resection. The subclavian artery is assessed, and if reconstruction is required, the proximal and distal aspects of the subclavian artery are exposed in preparation for repair. The distal subclavian artery can typically be elevated into the supraclavicular exposure to clamp the normal artery beyond the lesion; in some cases when the aneurysmal segment of subclavian artery extends underneath the clavicle, a second transverse infraclavicular incision is used to expose and control the distal subclavian artery. Following systemic anticoagulation, the subclavian artery is clamped, and the aneurysmal segment is excised. Arterial reconstruction is completed with an interposition bypass graft utilizing a prosthetic, autologous (e.g., reversed saphenous vein), or allograft (e.g., cryopreserved femoral artery or vein) as the conduit, with beveled end-to-end anastomoses.
Concomitant pectoralis minor tenotomy is performed for neurogenic TOS with brachial plexus compression at the subcoracoid space, or as an isolated procedure in selected patients with no supraclavicular findings. A vertical incision is made in the deltopectoral groove and carried through the subcutaneous tissues to the axillary space. The pectoralis major muscle is elevated, and the underlying pectoralis minor muscle is exposed. The pectoralis minor muscle tendon is encircled near its insertion on the coracoid process, with protection of the underlying neurovascular structures and the adjacent insertion of the short head of the biceps muscle, and the pectoralis minor tendon is divided with a cautery. The medial aspect of the pectoralis minor muscle is typically seen to retract away from the neurovascular structures and no further muscle resection is needed. The wound is closed in layers.
Upon completion of the procedure the pleural is inspected and if it has been opened, a small drain is placed into the pleural space and attached to suction. Alternatively, some surgeons will prefer to place a drain regardless of pleural entry. The incision is closed in layers.
Paraclavicular approach:[182]
General anesthesia is used and may be complemented by placement of regional anesthesia blocks prior to or after operation..[175][176][177][178][179][180][181]
The patient is positioned supine with the arm resting along the side. An initial venogram is typically performed following placement of a vascular sheath in the basilic vein. The procedure begins with supraclavicular decompression similar to that described above, with resection of the anterior and middle scalene muscles and mobilization of the subclavian artery and brachial plexus nerves. Once the posterior first rib has been divided at the costotransverse joint, a second transverse incision is made in the infraclavicular space immediately over the anteromedial first rib. Identification of the first rib can be facilitated by placing digital pressure on the divided end of the posterior first rib from the supraclavicular exposure. The pectoralis major muscle is separated without dividing muscle fibers to expose the first rib. The intercostal muscle attached to the lower edge of the first rib is divided and the undersurface of the entire rib is mobilized. The medial first rib is divided at the edge of the sternum using a bone rongeur. The subclavius muscle and costoclavicular ligament are then divided from the superior edge of the first rib, and once free of attachments the entire first rib is removed as a specimen. The axillary vein is then exposed along the lower aspect of the clavicle and dissected from surrounding fibrous tissues. If more lateral exposure of the axillary vein is needed, the infraclavicular incision can be extended laterally, and the pectoralis minor muscle divided to expose the axillary vein within the subcoracoid space. Returning to the supraclavicular exposure, the subclavian vein is exposed and dissected from surrounding fibrous tissue attachments. As the subclavian vein is mobilized it may be useful to divide 1-2 collateral veins that extend to the clavicle. The subclavian vein is then fully mobilized to its junction with the internal jugular and innominate veins, and once external venolysis is completed, the vein is assessed by inspection and palpation. An intraoperative venogram is performed to determine if direct subclavian vein repair is needed.
For subclavian vein reconstruction the patient is anticoagulated, and clamps are placed on the innominate, internal jugular, and subclavian veins from the supraclavicular exposure (although it is often helpful to place the innominate vein clamp from the infraclavicular exposure). For localized stenosis of the subclavian vein, a longitudinal venotomy is created in the normal distal subclavian vein and extended through the stenosis to the normal jugular-innominate vein junction. The vein is opened, and any thrombus or intraluminal fibrous webs are removed. A wide patch angioplasty is then created with either bovine pericardium or cryopreserved femoral vein. For longer occlusions of the subclavian vein, the normal jugular-innominate vein junction is opened and the venotomy is used to attach the venous conduit (typically using cryopreserved femoral vein) in an end-to-side anastomosis. The venous conduit is passed to the distal subclavian vein or axillary vein and a longitudinal venotomy is created in the normal distal vein at the site selected for inflow. The conduit is beveled and attached to the distal venotomy in a longitudinal anastomosis. Once clamps are removed a completion venogram is performed through the previously placed vascular sheath. Upon completion of the procedure the pleural is opened and a small drain is placed into the pleural space and attached to suction. The incisions are closed in layers.
Use of this content is subject to our disclaimer