The treatment of leishmaniasis is complex and, after discussing the primary drug options more generally, discussion is grouped by major clinical syndromes. It is important to recognise that the available literature on treatment varies in methodology with relatively few well-controlled clinical trials and a diversity of treatment endpoints.
For all types of leishmaniasis, parasite and host factors can influence treatment outcomes. Not only can treatment outcomes vary by species but there can be differences seen within a single species from different geographical regions. For example, the response to treatment of visceral leishmaniasis (VL) caused by Leishmania donovani varies significantly between South Asia and East Africa, with liposomal amphotericin-B being preferred in the former and pentavalent antimonial compounds retaining higher efficacy in the latter.[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
Host factors that influence the response to treatment include nutritional status and the presence of immunocompromise, such as HIV/AIDS.
In addition to treating the parasite, consideration has to be given to managing co-infections and/or supportive care, particularly in VL. The goal of therapy in all forms of leishmaniasis is clinical cure, recognising that Leishmania parasites likely persist after treatment.[108]Martínez-Valencia AJ, Daza-Rivera CF, Rosales-Chilama M, et al. Clinical and parasitological factors in parasite persistence after treatment and clinical cure of cutaneous leishmaniasis. PLoS Negl Trop Dis. 2017 Jul 13;11(7):e0005713.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5526576
http://www.ncbi.nlm.nih.gov/pubmed/28704369?tool=bestpractice.com
[109]Dereure J, Duong Thanh H, Lavabre-Bertrand T, et al. Visceral leishmaniasis: persistence of parasites in lymph nodes after clinical cure. J Infect. 2003 Jul;47(1):77-81.
http://www.ncbi.nlm.nih.gov/pubmed/12850167?tool=bestpractice.com
General considerations for choosing therapy include regional efficacy, safety, availability, and cost, with the latter two often driving management decisions in low-resource settings.
Anti-leishmanial drugs
Amphotericin-B
Liposomal amphotericin-B is the preferred formulation for the treatment of leishmaniasis, when available. Its use is limited by cost, availability, and the requirement of a cold chain. It is approved in Europe and the US for VL; use in other types of leishmaniasis is off-label. Liposomal amphotericin-B is generally well tolerated with adverse effects including infusion-related reactions (e.g., chills, fever), nausea, vomiting, hypokalaemia, and renal insufficiency.[110]Berman JD. U.S Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis. 1999 Jan;28(1):49-51.
https://academic.oup.com/cid/article/28/1/49/302484?login=false
http://www.ncbi.nlm.nih.gov/pubmed/10391695?tool=bestpractice.com
[111]Berman JD, Badaro R, Thakur CP, et al. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull World Health Organ. 998;76(1):25-32.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2305623
http://www.ncbi.nlm.nih.gov/pubmed/9615494?tool=bestpractice.com
[112]Bern C, Adler-Moore J, Berenguer J, et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis. 2006 Oct 1;43(7):917-24.
http://cid.oxfordjournals.org/content/43/7/917.full
http://www.ncbi.nlm.nih.gov/pubmed/16941377?tool=bestpractice.com
Amphotericin deoxycholate is an option if the liposomal formulation is not available; adverse effects are similar but more frequent and severe, and administration is more complicated.[113]Olliaro PL, Guerin PJ, Gerstl S, et al. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980-2004. Lancet Infect Dis. 2005 Dec;5(12):763-74.
http://www.ncbi.nlm.nih.gov/pubmed/16310148?tool=bestpractice.com
[114]Sundar S, Mehta H, Suresh AV, et al. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis. 2004 Feb 1;38(3):377-83.
http://cid.oxfordjournals.org/content/38/3/377.full
http://www.ncbi.nlm.nih.gov/pubmed/14727208?tool=bestpractice.com
Other lipid formulations of amphotericin-B, if available, have been less rigorously studied and appear to be inferior to liposomal amphotericin-B.[115]Yardley V, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000 Feb;13(4):243-8.
http://www.ncbi.nlm.nih.gov/pubmed/10755238?tool=bestpractice.com
Amphotericin-B is the only anti-leishmanial with safety data in pregnancy.
Miltefosine
Miltefosine is the only oral anti-leishmanial available.
Use of miltefosine is limited by:
High risk for teratogenicity in pregnancy. Women require a negative pregnancy test and effective contraception during treatment and for at least 5 months after completion of treatment
Significant gastrointestinal adverse effects including nausea, vomiting, and abdominal pain
Natural or emerging resistance in some regions.[116]Carnielli JBT, Crouch K, Forrester S, et al. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine. 2018 Oct;36:83-91.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6197651
http://www.ncbi.nlm.nih.gov/pubmed/30268832?tool=bestpractice.com
[117]Tiwary P, Kumar D, Sundar S. Identification and functional validation of a biomarker for the diagnosis of miltefosine relapse during visceral leishmaniasis. Am J Trop Med Hyg. 2018 Feb;98(2):492-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6197651
http://www.ncbi.nlm.nih.gov/pubmed/29280431?tool=bestpractice.com
Miltefosine is approved in the US (but not in Europe) for use in:
VL caused by Leishmania donovani
Cutaneous leishmaniasis (CL) due to Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis
Mucosal leishmaniasis (ML) due to Leishmania braziliensis.
Pentavalent antimonial compounds
Specific drugs include sodium stibogluconate and meglumine antimonate. Branded products are generally preferred due to more reliable content and less toxicity.[121]Sundar S, Chakravarty J. Antimony toxicity. Int J Environ Res Public Health. 2010 Dec;7(12):4267-77.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3037053
http://www.ncbi.nlm.nih.gov/pubmed/21318007?tool=bestpractice.com
In the US, there are no Food and Drug Administration (FDA)-approved pentavalent antimonial compounds currently available. In Europe, sodium stibogluconate is licensed for use in all major forms of leishmaniasis.
Significant regional differences in efficacy have been seen, with a high level of resistance in South Asia.[122]Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001 Nov;6(11):849-54.
https://onlinelibrary.wiley.com/doi/full/10.1046/j.1365-3156.2001.00778.x
http://www.ncbi.nlm.nih.gov/pubmed/11703838?tool=bestpractice.com
Paromomycin
Paromomycin is an aminoglycoside that can be administered topically in CL or intramuscularly in VL, and is often used in combination with another agent.
Neither intramuscular nor topical preparations of paromomycin are approved for use in the US or Europe. Oral preparations in the US are approved for other indications and can be used to compound topical ointments, but this use is off-label.
When used topically, paromomycin can cause mild to moderate local-site reactions including redness, pain, swelling, burning, and itching. Adverse effects with systemic paromomycin include significant injection-site pain, elevated aminotransferases, renal insufficiency, and reversible ototoxicity.
Safety data are lacking for paromomycin in pregnancy.
Pentamidine
Use is generally limited to specific situations in CL and secondary prophylaxis among immunocompromised patients.
Pentamidine is available in the US, but use is off-label in leishmaniasis. It is approved in Europe for the treatment of CL.
Adverse effects are significant and include diabetes mellitus, pancreatitis, gastrointestinal symptoms, hypotension, QT prolongation, electrolyte disturbances, nephrotoxicity, hepatotoxicity, cytopenias, and rhabdomyolysis. These effects are mitigated when drug is administered intralesionally for cutaneous leishmaniasis.
There are no data on the safety of pentamidine in pregnancy.
Other drugs such as azole antifungals may have a role in some specific situations.
Cutaneous leishmaniasis: general principles
The appropriate management is individualised for each CL patient. The Infectious Diseases Society of America (IDSA)/American Society of Tropical Medicine and Hygiene (ASTMH) guidelines classify cutaneous leishmaniasis as either simple or complex.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
Simple CL:
No mucosal involvement and parasite species not associated with ML
≤4 lesions <1 cm
Location feasible for local therapy and/or non-exposed skin (not cosmetically important)
Immunocompetent host
Lesions resolving without therapy.
Complex CL:
Parasite species caused by species associated with ML
Local subcutaneous nodules
Large regional adenopathy
>4 lesions generally >1 cm
Individual lesion ≥5 cm
Size or location makes local therapy infeasible and/or lesion of the face, fingers, toes, joints, or genitalia
Immunocompromised host
Failure of local therapy
Unusual syndromes (leishmaniasis recidivans, diffuse cutaneous leishmaniasis, or disseminated leishmaniasis).
In high-resource settings, parasitological diagnosis with species-level identification is optimal to guide management and should be obtained. In low-resource settings, attempt should still be made to confirm the diagnosis to the extent possible due to the significant adverse effects associated with treatment.[123]Croft SL, Yardley V. Chemotherapy of leishmaniasis. Curr Pharm Des. 2002;8(4):319-42.
http://www.ncbi.nlm.nih.gov/pubmed/11860369?tool=bestpractice.com
[124]Amato VS, Tuon FF, Siqueira AM, et al. Treatment of mucosal leishmaniasis in Latin America: systematic review. Am J Trop Med Hyg. 2007 Aug;77(2):266-74.
http://www.ncbi.nlm.nih.gov/pubmed/17690398?tool=bestpractice.com
[125]Oliveira LF, Schubach AO, Martins MM, et al. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Tropica. 2011 May;118(2):87-96.
http://www.ncbi.nlm.nih.gov/pubmed/21420925?tool=bestpractice.com
In cases where the species cannot be determined, knowledge of regional (old world versus new world) and local prevalence patterns may help in guiding therapy. For example, a patient with CL from the 'mucosal belt' of Bolivia, Brazil, and Peru may be at higher risk of mucosal spread and so consideration might be given to monitoring patients without a clear species-specific diagnosis for the development of mucosal disease after management of the primary skin lesion(s).
Simple CL may require only watchful waiting or, to accelerate cure, can be managed with local therapies or potentially azole antifungals. Complex CL generally requires systemic therapy to prevent disability and social stigma, and/or to prevent parasite dissemination (e.g., mucosal leishmaniasis [ML]) or relapse.
For all cases of ulcerative CL, care should be taken to ensure proper wound management including washing of ulcer(s) and applying barrier ointment. The presence of warmth, redness, pain, and/or purulent discharge should prompt for the evaluation and management of a secondary bacterial infection. Evidence of healing often begins with flattening, and large ulcers may not be completely healed by the end of therapy.
CDC: parasites – leishmaniasis
Opens in new window
Treatment recommendations for specific situations are given below; however, most trials on the treatment of CL have been poorly designed and reported, resulting in a lack of clear evidence for potentially beneficial treatments. There is a need for large, well-conducted studies that evaluate long-term effects of current therapies, and evaluate recurrence rates of cutaneous leishmaniasis and its progression to mucosal disease.[126]Heras-Mosteiro J, Monge-Maillo B, Pinart M, et al. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2017 Nov 17;11(11):CD005067.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005067.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/29149474?tool=bestpractice.com
[127]Pinart M, Rueda JR, Romero GA, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD004834.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004834.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/32853410?tool=bestpractice.com
Cutaneous leishmaniasis: specific treatment
Simple cutaneous leishmaniasis
For simple CL, options include watchful waiting, local therapies, or less toxic systemic medications.
Most cases of CL, particularly among Old World species, will resolve spontaneously within 18 months; therefore, it is important to decide whether or not treatment is truly indicated.[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
[128]Morizot G, Kendjo E, Mouri O, et al. Travelers with cutaneous leishmaniasis cured without systemic therapy. Clin Infect Dis. 2013 Aug;57(3):370-80.
https://academic.oup.com/cid/article/57/3/370/461927
http://www.ncbi.nlm.nih.gov/pubmed/23633111?tool=bestpractice.com
This decision must be individualised considering factors such as whether healing has already begun, impairments of wound healing, risk of secondary infections, patient preference, whether there is evidence of impaired cell-mediated immunity, and availability/expertise in treatment.
Among those who undergo treatment, options for local therapy include cryotherapy, thermotherapy, and topical or intralesional medications. Before any local therapy is started, any crust should be removed, if present.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
Cryotherapy: involves freezing the lesion and surrounding 1 to 2 mm of healthy skin with liquid nitrogen until it is white in appearance, allowing the area to thaw, and freezing again. The IDSA/ASTMH guidelines recommend a cryotherapy session every 3 weeks up to three times total.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
The per lesion efficacy of cryotherapy is 67% across all regions, and is statistically the same as intralesional antimonial compounds.[129]López-Carvajal L, Cardona-Arias JA, Zapata-Cardona MI, et al. Efficacy of cryotherapy for the treatment of cutaneous leishmaniasis: meta-analyses of clinical trials. BMC Infect Dis. 2016 Jul 26;16:360.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4960741
http://www.ncbi.nlm.nih.gov/pubmed/27456008?tool=bestpractice.com
Adverse effects include transient pain and redness as well as hypopigmentation.
Thermotherapy: utilises heat administered to the lesion(s), often in a single treatment, resulting in a second-degree burn and requiring local anaesthesia. A meta-analysis showed 73% efficacy across all regions, equivalent to systemic antimonial therapy but with fewer adverse effects.[130]Cardona-Arias JA, Vélez ID, López-Carvajal L. Efficacy of thermotherapy to treat cutaneous leishmaniasis: a meta-analysis of controlled clinical trials. 2015 May 26;10(5):e0122569.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0122569
http://www.ncbi.nlm.nih.gov/pubmed/26009885?tool=bestpractice.com
Efficacy may be even higher in the Old World, with cure rates as high as 94% in Leishmania tropica.[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
Adverse effects include cellulitis, redness, and pain at the treatment site, and US guidelines recommend utilising topical antibiotics post-procedurally for several days.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
This treatment modality requires the use of special equipment and operator training on this equipment, which limits its broad applicability.
Topical paromomycin: the best studied topical medication for the treatment of CL. The efficacy varies in the literature with the precise formulation; therefore, commercial and compounded versions may not be equivalent to what is reported. Paromomycin may be used alone or in combination with methylbenzethonium chloride, urea, or gentamicin.[126]Heras-Mosteiro J, Monge-Maillo B, Pinart M, et al. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2017 Nov 17;11(11):CD005067.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005067.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/29149474?tool=bestpractice.com
In the Old World disease with ulcerative Leishmania major an overall cure rate of approximately 80% was seen (with or without the addition of gentamicin) compared with 57% in the vehicle only arm.[131]Ben Salah A, Ben Messaoud N, Guedri E, et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 2013 Feb 7;368(6):524-32.
http://www.ncbi.nlm.nih.gov/pubmed/23388004?tool=bestpractice.com
In two studies in the New World among lesions caused by L panamensis, L guyanensis, or L braziliensis, similar paromomycin products had cure rates of 77% to 79%.[132]Sosa N, Pascale JM, Jiménez AI, et al. Topical paromomycin for New World cutaneous leishmaniasis. PLoS Negl Trop Dis. 2019 May 2;13(5):e0007253.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6497224
http://www.ncbi.nlm.nih.gov/pubmed/31048871?tool=bestpractice.com
[133]Soto J, Soto P, Ajata A, et al. Topical 15% paromomycin-aquaphilic for Bolivian Leishmania braziliensis cutaneous leishmaniasis: a randomized, placebo-controlled trial. Clin Infect Dis. 2019 Feb 15;68(5):844-9.
https://academic.oup.com/cid/article/68/5/844/5107841
http://www.ncbi.nlm.nih.gov/pubmed/30260376?tool=bestpractice.com
Older studies in the New World have shown lower rates of efficacy (approximately 50%) with various different paromomycin formulations; however, the cure rates with systemic pentavalent antimonial compounds in these studies were similar (74%) to topical paromomycin in newer studies.[134]Kim DH, Chung HJ, Bleys J, et al. Is paromomycin an effective and safe treatment against cutaneous leishmaniasis? A meta-analysis of 14 randomized controlled trials. PLoS Negl Trop Dis. 2009;3(2):e381.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2637543
http://www.ncbi.nlm.nih.gov/pubmed/19221595?tool=bestpractice.com
In Panama, 3% of patients treated with topical paromomycin developed mucosal disease that required systemic treatment.[132]Sosa N, Pascale JM, Jiménez AI, et al. Topical paromomycin for New World cutaneous leishmaniasis. PLoS Negl Trop Dis. 2019 May 2;13(5):e0007253.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6497224
http://www.ncbi.nlm.nih.gov/pubmed/31048871?tool=bestpractice.com
The risk of mucosal disease among patients with Viannia subgenus parasites remains a significant concern warranting close monitoring if topical treatment is chosen versus opting for systemic therapy initially.
Intralesional therapy: involves injecting lesions with an anti-leishmanial drug, most commonly pentavalent antimonial compounds. Pain with injection can be significant. In the Old World, the best results are seen by combining intralesional sodium stibogluconate with cryotherapy, with cure rates of 89% to 91% reported.[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
While the evidence in the New World is less robust and concerns for ML remain, a meta-analysis showed pooled efficacy of 75%, but with a preference for meglumine antimonate (efficacy 82%) rather than sodium stibogluconate, which is preferred in the Old World.[135]Brito NC, Rabello A, Cota GF. Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: a systematic review. PLoS One. 2017 Sep 19;12(9):e0184777.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5604971
http://www.ncbi.nlm.nih.gov/pubmed/28926630?tool=bestpractice.com
Sodium stibogluconate is available for intralesional use in Europe, but is not available in the US. Pentamidine can also be used intralesionally.[136]Soto J, Paz D, Rivero D, et al. Intralesional pentamidine: a novel therapy for single lesions of Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2016 Apr;94(4):852-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4824229
http://www.ncbi.nlm.nih.gov/pubmed/26903605?tool=bestpractice.com
Other methodologies such as photodynamic therapy or CO₂ laser therapy are potential alternative local therapies, but limited evidence prevents making recommendations regarding their use. [Figure caption and citation for the preceding image starts]: Intralesional injection for the treatment of cutaneous leishmaniasisImage courtesy of the World Health Organization [Citation ends].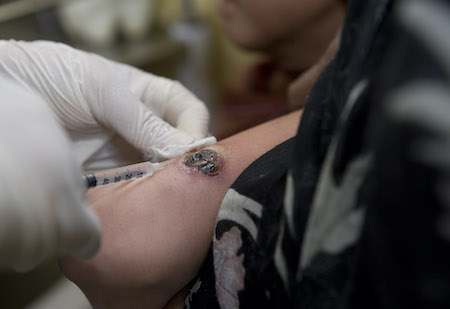
Generally, systemic therapy is reserved for complex disease, but azole antifungal therapy may be an option in simple CL.
Fluconazole, itraconazole, and ketoconazole have been evaluated and are available globally, although use in CL is off-label in both the US and Europe.
All have gastrointestinal adverse effects and a risk of hepatotoxicity, but ketoconazole carries a warning related to the risk of life-threatening hepatotoxicity and QT prolongation that could lead to fatal arrhythmias.
A systematic review did not find enough evidence to recommend any of the azole antifungals routinely, but in the New World pooled efficacy for ketoconazole in the treatment of Leishmania mexicana was as high as 89%, suggesting this as a potential option in Mexico and parts of Central America with appropriate monitoring of adverse effects.[137]Galvão EL, Rabello A, Cota GF. Efficacy of azole therapy for tegumentary leishmaniasis: a systematic review and meta-analysis. PLoS One. 2017 Oct 9;12(10):e0186117.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5633178
http://www.ncbi.nlm.nih.gov/pubmed/29016694?tool=bestpractice.com
Complex cutaneous leishmaniasis
In complex CL, systemic therapy is preferred due to the extent or location of the lesion(s), prior failure of local therapy, and/or the risk of dissemination.
Miltefosine: a more recent option for CL. It is approved for Viannia subgenus parasites in the New World, but even in that context there is variability regionally. Cure rates in Brazil, Bolivia, and Colombia range from 71% to 91% for Viannia species, but in Guatemala the cure rate was only 33% for L braziliensis.[138]Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019 Jul;20(10):1251-65.
http://www.ncbi.nlm.nih.gov/pubmed/31063412?tool=bestpractice.com
Pentavalent antimonial compounds (sodium stibogluconate, meglumine antimonate): generally effective but less favourable due to the associated toxicity and duration of treatment. In Old World disease, cure rates range as high as 98% for L major and as low as 41% for L tropica. In the New World, efficacy ranges from 77% to 90%, but significant levels of treatment failure are seen among parasites of the Viannia subgenus, especially L braziliensis, and also in certain geographical regions such as L mexicana in Guatemala, or among Leishmania species in the southern jungle regions of Peru bordering with Bolivia.[138]Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019 Jul;20(10):1251-65.
http://www.ncbi.nlm.nih.gov/pubmed/31063412?tool=bestpractice.com
Liposomal amphotericin-B: likely effective for complex CL, but data are limited to observational studies and several small trials. Retrospective data from various regions range from 46% to 84% cured, but small trials in Bolivia had rates of 85% to 100%.[138]Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019 Jul;20(10):1251-65.
http://www.ncbi.nlm.nih.gov/pubmed/31063412?tool=bestpractice.com
Pentamidine: has a limited role in treating CL in the New World caused by L guyanensis in French Guiana and Suriname with cure rates generally of 84% to 90% reported.[139]Roussel M, Nacher M, Frémont G, et al. Comparison between one and two injections of pentamidine isethionate, at 7 mg/kg in each injection, in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2006 Jun;100(4):307-14.
http://www.ncbi.nlm.nih.gov/pubmed/16762111?tool=bestpractice.com
[140]Lai A Fat EJ, Vrede MA, Soetosenojo RM, et al. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int J Dermatol. 2002 Nov;41(11):796-800.
http://www.ncbi.nlm.nih.gov/pubmed/12453009?tool=bestpractice.com
Intravenous injection is preferred over intramuscular injection as increased efficacy is seen with intravenous administration (85% cure with intravenous versus 51% with intramuscular).[141]Christen JR, Bourreau E, Demar M, et al. Use of the intramuscular route to administer pentamidine isethionate in Leishmania guyanensis cutaneous leishmaniasis increases the risk of treatment failure. Travel Med Infect Dis. 2018 Jul - Aug;24:31-6.
http://www.ncbi.nlm.nih.gov/pubmed/29482012?tool=bestpractice.com
Another potential use is intralesional pentamidine in Bolivia with a reported efficacy of 73%, but this may not impact the risk of mucosal spread.[136]Soto J, Paz D, Rivero D, et al. Intralesional pentamidine: a novel therapy for single lesions of Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2016 Apr;94(4):852-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4824229
http://www.ncbi.nlm.nih.gov/pubmed/26903605?tool=bestpractice.com
However, combination of this same regimen with 28 days of miltefosine resulted in 92% cure rates and has the advantage of systemic treatment, albeit with increased duration and cost.[142]Soto J, Soto P, Ajata A, et al. Miltefosine combined with intralesional pentamidine for Leishmania braziliensis cutaneous leishmaniasis in Bolivia. Am J Trop Med Hyg. 2018 Nov;99(5):1153-5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6221230
http://www.ncbi.nlm.nih.gov/pubmed/30255833?tool=bestpractice.com
In other settings, the adverse effects and relatively lower efficacy of pentamidine typically preclude its use. This may be due in part to underdosing based on the salt (isethionate) versus the base.[143]Dorlo TP, Kager PA. Pentamidine dosage: a base/salt confusion. PLoS Negl Trop Dis. 2008 May 28;2(5):e225.
https://www.doi.org/10.1371/journal.pntd.0000225
http://www.ncbi.nlm.nih.gov/pubmed/18509543?tool=bestpractice.com
The treatment of other complex forms of CL including disseminated leishmaniasis, diffuse cutaneous leishmaniasis, and leishmaniasis recidivans should be guided by experts as treatment can be challenging and data are limited.
Liposomal amphotericin-B has been used with reasonable success in disseminated leishmaniasis (cure rate of 75%).[144]Machado PR, Rosa ME, Guimarães LH, et al. Treatment of disseminated leishmaniasis with liposomal amphotericin B. Clin Infect Dis. 2015 Sep 15;61(6):945-9.
https://academic.oup.com/cid/article/61/6/945/450907
http://www.ncbi.nlm.nih.gov/pubmed/26048961?tool=bestpractice.com
For diffuse cutaneous leishmaniasis, the Pan American Health Organization recommends treatment in a referral centre and lists pentavalent antimonial compounds, pentamidine, or miltefosine as potential options.[145]Pan American Health Organization. Guideline for the treatment of Leishmaniasis in the Americas. Second edition. Sep 2022 [internet publication].
https://iris.paho.org/handle/10665.2/56120
However, cure in these patients can be challenging to achieve.
Leishmaniasis recidivans can be frustrating to treat.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
One potential regimen combines a pentavalent antimonial compound with allopurinol for 30 days.[146]Esfandiarpour I, Dabiri SH. Treatment of cutaneous leishmaniasis recidivans with a combination of allopurinol and meglumine antimoniate: a clinical and histologic study. Int J Dermatol. 2007 Aug;46(8):848-52.
http://www.ncbi.nlm.nih.gov/pubmed/17651170?tool=bestpractice.com
Liposomal amphotericin and miltefosine are often used.
Pregnant patients
Treatment of simple and complex cutaneous disease in pregnant patients is highly individualised. If possible, treatment should be deferred until after pregnancy, even exophytic lesions; however, there may be an increased risk of preterm birth and stillbirth in untreated CL patients.[147]Morgan DJ, Guimaraes LH, Machado PR, et al. Cutaneous leishmaniasis during pregnancy: exuberant lesions and potential fetal complications. Clin Infect Dis. 2007 Aug 15;45(4):478-82.
https://academic.oup.com/cid/article/45/4/478/426772
http://www.ncbi.nlm.nih.gov/pubmed/17638198?tool=bestpractice.com
Theoretical concerns for transplacental transmission of CL exist based on studies in mice, but this has not been reported in humans.[148]Avila-García M, Mancilla-Ramírez J, Segura-Cervantes E, et al. Transplacental transmission of cutaneous Leishmania mexicana strain in BALB/c mice. Am J Trop Med Hyg. 2013 Aug;89(2):354-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741259
http://www.ncbi.nlm.nih.gov/pubmed/23798582?tool=bestpractice.com
Local therapies are generally preferred to systemic therapies if possible, noting that intralesional therapies can lead to systemic absorption.
Amphotericin-B is the preferred agent if systemic therapy is required due to its relative safety compared with other options.
Pentavalent antimonial compounds have increased risks of miscarriage and early delivery, generally precluding their use during pregnancy.
Mucosal leishmaniasis
Patients with ML should always receive treatment with systemic therapy. Robust data are limited and treatment efficacy varies, requiring an individualised approach to therapy. Options for therapy include pentavalent antimonial compounds, amphotericin-B formulations, miltefosine, and pentamidine. The Pan American Health Organization classifies the evidence of all options as low or very low, but gives its strongest recommendation to pentavalent antimonial compounds.[145]Pan American Health Organization. Guideline for the treatment of Leishmaniasis in the Americas. Second edition. Sep 2022 [internet publication].
https://iris.paho.org/handle/10665.2/56120
[149]Pan American Health Organization. Manual of procedures for leishmaniases surveillance and control in the Americas. Dec 2019 [internet publication].
https://iris.paho.org/handle/10665.2/51838
The US guidelines do not stratify among the alternatives other than classifying pentamidine as a lesser alternative.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
Pentavalent antimonial compounds are a reasonable first choice in patients able to tolerate the course. Efficacy varies from 58% with sodium stibogluconate to 88% with meglumine antimonate. Some studies have added adjuncts such as pentoxifylline or interferon-gamma, but evidence for this is weak.[124]Amato VS, Tuon FF, Siqueira AM, et al. Treatment of mucosal leishmaniasis in Latin America: systematic review. Am J Trop Med Hyg. 2007 Aug;77(2):266-74.
http://www.ncbi.nlm.nih.gov/pubmed/17690398?tool=bestpractice.com
Amphotericin-B formulations are an alternative, particularly for treatment failure, relapse, or intolerance of pentavalent antimonial compounds. Liposomal amphotericin-B has less data, but is preferred when available in light of better safety and tolerability.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
In a retrospective series from Brazil, the cure rate was 93% for patients treated with liposomal amphotericin-B.[150]Cunha MA, Leão AC, de Cassia Soler R, et al. Efficacy and safety of liposomal amphotericin B for the treatment of mucosal leishmaniasis from the New World: a retrospective study. Am J Trop Med Hyg. 2015 Dec;93(6):1214-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4674237
http://www.ncbi.nlm.nih.gov/pubmed/26483120?tool=bestpractice.com
If this treatment fails, miltefosine can be used, but cure rates are low apart from in clinically mild disease.[151]Soto J, Toledo J, Valda L, et al. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin Infect Dis. 2007 Feb 1;44(3):350-6.
https://academic.oup.com/cid/article/44/3/350/312370
http://www.ncbi.nlm.nih.gov/pubmed/17205440?tool=bestpractice.com
Pregnant patients
Visceral leishmaniasis: general principles
The main objective of treatment is prevention of death and achievement of long-term clinical cure. Eradication of parasites is unlikely in most patients, as evidenced by disease reactivation years after initial clinical cure and by the post-treatment persistence of parasites in a significant proportion of patients when highly sensitive detection methods (e.g., polymerase chain reaction) are used.[82]Cruz I, Chicharro C, Nieto J, et al. Comparison of new diagnostic tools for management of pediatric Mediterranean visceral leishmaniasis. J Clin Microbiol. 2006 Jul;44(7):2343-7.
https://journals.asm.org/doi/10.1128/JCM.02297-05?url_ver=Z39.88-2003
http://www.ncbi.nlm.nih.gov/pubmed/16825347?tool=bestpractice.com
[152]Osman OF, Oskam L, Zijlstra EE, et al. Use of the polymerase chain reaction to assess the success of visceral leishmaniasis treatment. Trans R Soc Trop Med Hyg. 1998 Jul-Aug;92(4):397-400.
http://www.ncbi.nlm.nih.gov/pubmed/9850390?tool=bestpractice.com
As in CL, there are parasite factors (often corresponding to species and/or geographical region) and host factors that should be considered when determining a treatment course for an individual. All patients with symptomatic disease should be treated. It is unknown whether the treatment of latent VL infection would prevent the development of symptomatic disease and it is not recommended, especially in light of the toxicities of available treatments.
In addition to the treatment of Leishmania, management must address frequent complications such as volume depletion, malnutrition, anaemia, and concomitant bacterial infections (e.g., pneumonia) or parasitic infections (e.g., malaria).
Visceral leishmaniasis: treatment in immunocompetent patients
Amphotericin-B formulations
Outside of East Africa, liposomal amphotericin-B is the treatment of choice, with clinical cure in >90% with varying regimens.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
[138]Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019 Jul;20(10):1251-65.
http://www.ncbi.nlm.nih.gov/pubmed/31063412?tool=bestpractice.com
For L donovani in India, liposomal amphotericin-B was demonstrated to be reasonably safe and had a 96% cure rate in clinical trials.[153]Sundar S, Chakravarty J, Agarwal D, et al. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010 Feb 11;362(6):504-12.
https://www.doi.org/10.1056/NEJMoa0903627
http://www.ncbi.nlm.nih.gov/pubmed/20147716?tool=bestpractice.com
Multiple subsequent studies including over 2500 patients treated with liposomal amphotericin-B across South Asia have shown similar or higher efficacy.[154]Lucero E, Collin SM, Gomes S, et al. Effectiveness and safety of short course liposomal amphotericin B (AmBisome) as first line treatment for visceral leishmaniasis in Bangladesh. PLoS Negl Trop Dis. 2015 Apr 2;9(4):e0003699.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4383421
http://www.ncbi.nlm.nih.gov/pubmed/25837313?tool=bestpractice.com
[155]Goswami RP, Goswami RP, Das S, et al. Short-course treatment regimen of Indian visceral leishmaniasis with an Indian liposomal amphotericin B preparation (Fungisome™). Am J Trop Med Hyg. 2016 Jan;94(1):93-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4710453
http://www.ncbi.nlm.nih.gov/pubmed/26526926?tool=bestpractice.com
[156]Pandey K, Pal B, Siddiqui NA, et al. Efficacy and safety of liposomal amphotericin B for visceral leishmaniasis in children and adolescents at a tertiary care center in Bihar, India. Am J Trop Med Hyg. 2017 Nov;97(5):1498-502.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5817748
http://www.ncbi.nlm.nih.gov/pubmed/29016288?tool=bestpractice.com
[157]Rahman R, Goyal V, Haque R, et al. Safety and efficacy of short course combination regimens with AmBisome, miltefosine and paromomycin for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Negl Trop Dis. 2017 May 30;11(5):e0005635.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5466346
http://www.ncbi.nlm.nih.gov/pubmed/28558062?tool=bestpractice.com
[158]Goyal V, Mahajan R, Pandey K, et al. Field safety and effectiveness of new visceral leishmaniasis treatment regimens within public health facilities in Bihar, India. PLoS Negl Trop Dis. 2018 Oct 22;12(10):e0006830.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6197645
http://www.ncbi.nlm.nih.gov/pubmed/30346949?tool=bestpractice.com
In East Africa, higher doses of liposomal amphotericin-B are required to treat L donovani and have achieved a clinical cure in >90% despite most of these patients being complex (e.g., pregnancy, relapse, advanced disease, or extremes of age).[159]Copeland NK, Aronson NE. Leishmaniasis: treatment updates and clinical practice guidelines review. Curr Opin Infect Dis. 2015 Oct;28(5):426-37.
http://www.ncbi.nlm.nih.gov/pubmed/26312442?tool=bestpractice.com
[160]Gebreyohannes EA, Bhagvathula AS, Abegaz TM, et al. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and meta-analysis. Infect Dis Poverty. 2018 Oct 19;7(1):108.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6194743
http://www.ncbi.nlm.nih.gov/pubmed/30340519?tool=bestpractice.com
Leishmania infantum (also known as Leishmania chagasi) is also preferentially treated with liposomal amphotericin-B.[138]Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019 Jul;20(10):1251-65.
http://www.ncbi.nlm.nih.gov/pubmed/31063412?tool=bestpractice.com
Most efficacy data are from the Mediterranean region, but a study in Brazil supports the use of liposomal amphotericin-B over pentavalent antimonial compounds and modelling demonstrates the cost-effectiveness of this approach.[161]Romero GAS, Costa DL, Costa CHN, et al. Efficacy and safety of available treatments for visceral leishmaniasis in Brazil: a multicenter, randomized, open label trial. PLoS Negl Trop Dis. 2017 Jun 29;11(6):e0005706.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5507560
http://www.ncbi.nlm.nih.gov/pubmed/28662034?tool=bestpractice.com
[162]Assis TSM, Rabello A, Cota G, et al. Cost-effectiveness analysis of diagnostic-therapeutic strategies for visceral leishmaniasis in Brazil. Rev Soc Bras Med Trop. 2019 Apr 11;52:e20180272.
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822019000100638&lng=en&nrm=iso&tlng=en
http://www.ncbi.nlm.nih.gov/pubmed/30994802?tool=bestpractice.com
Combination therapy
The combination of pentavalent antimonial compounds with paromomycin is highly effective for the treatment of VL due to L donovani in East Africa, with cure rates of 90% to 95%.[160]Gebreyohannes EA, Bhagvathula AS, Abegaz TM, et al. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and meta-analysis. Infect Dis Poverty. 2018 Oct 19;7(1):108.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6194743
http://www.ncbi.nlm.nih.gov/pubmed/30340519?tool=bestpractice.com
[163]Kimutai R, Musa AM, Njoroge S, et al. Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral leishmaniasis in eastern Africa: results from a pharmacovigilance programme. Clin Drug Investig. 2017 Mar;37(3):259-72.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5315726
http://www.ncbi.nlm.nih.gov/pubmed/28066878?tool=bestpractice.com
This drug combination should not be used in patients >50 years of age or those with HIV due to decreased cure rates of 81% and 56%, respectively.[163]Kimutai R, Musa AM, Njoroge S, et al. Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral leishmaniasis in eastern Africa: results from a pharmacovigilance programme. Clin Drug Investig. 2017 Mar;37(3):259-72.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5315726
http://www.ncbi.nlm.nih.gov/pubmed/28066878?tool=bestpractice.com
As parenteral paromomycin is not available in Europe or the US, it is unusual to see this regimen used outside of East Africa.
In South Asia, the combinations of liposomal amphotericin-B with short courses of miltefosine or paromomycin, or miltefosine with paromomycin, are highly effective alternatives to amphotericin-B.[157]Rahman R, Goyal V, Haque R, et al. Safety and efficacy of short course combination regimens with AmBisome, miltefosine and paromomycin for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Negl Trop Dis. 2017 May 30;11(5):e0005635.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5466346
http://www.ncbi.nlm.nih.gov/pubmed/28558062?tool=bestpractice.com
[158]Goyal V, Mahajan R, Pandey K, et al. Field safety and effectiveness of new visceral leishmaniasis treatment regimens within public health facilities in Bihar, India. PLoS Negl Trop Dis. 2018 Oct 22;12(10):e0006830.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6197645
http://www.ncbi.nlm.nih.gov/pubmed/30346949?tool=bestpractice.com
[164]Sundar S, Sinha PK, Rai M, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011 Feb 5;377(9764):477-86.
http://www.ncbi.nlm.nih.gov/pubmed/21255828?tool=bestpractice.com
Other monotherapy options
Miltefosine monotherapy is an alternative option. In South Asia, initial results were excellent, but after 10 years of use a decline in efficacy was observed and now use is generally as an alternative or in combination with other drugs. Effectiveness of monotherapy in East Africa and South America is also suboptimal.[138]Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019 Jul;20(10):1251-65.
http://www.ncbi.nlm.nih.gov/pubmed/31063412?tool=bestpractice.com
Pentavalent antimonial compounds can be used for L donovani in East Africa, or in L infantum with decent efficacy, but they are considered second-line when other options are not available. Drug resistance in South Asia precludes their use in the subcontinent.[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
While paromomycin monotherapy has been demonstrated as 95% to 98% effective when compared with amphotericin-B or as a single arm in South Asia in limited studies, other safer and equally efficacious options make this a lower-tier option even in areas where it is available.[165]Sundar S, Jha TK, Thakur CP, et al. Injectable paromomycin for visceral leishmaniasis in India. N Engl J Med. 2007 Jun 21;356(25):2571-81.
https://www.nejm.org/doi/10.1056/NEJMoa066536?url_ver=Z39.88-2003
http://www.ncbi.nlm.nih.gov/pubmed/17582067?tool=bestpractice.com
[166]Jamil KM, Haque R, Rahman R, et al. Effectiveness study of paromomycin IM injection (PMIM) for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Negl Trop Dis. 2015 Oct 23;9(10):e0004118.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4619770
http://www.ncbi.nlm.nih.gov/pubmed/26496648?tool=bestpractice.com
Patients who do not respond to, or who relapse after, initial therapy should receive full treatment with an anti-leishmanial drug (or a combination of anti-leishmanial drugs) from a different class. However, if the initial treatment was with liposomal amphotericin-B, retreatment with amphotericin-B, potentially at higher total doses, is appropriate.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[107]Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-70.
http://www.ncbi.nlm.nih.gov/pubmed/30126638?tool=bestpractice.com
Pregnant patients
Treatment is essential, because untreated VL can be fatal to both mother and fetus.
Limited data suggest that liposomal amphotericin-B is a safe and effective treatment option.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[119]Pagliano P, Carannante N, Rossi M, et al. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. J Antimicrob Chemother. 2005 Feb;55(2):229-33.
https://academic.oup.com/jac/article/55/2/229/856828?login=false
http://www.ncbi.nlm.nih.gov/pubmed/15649998?tool=bestpractice.com
Use of pentavalent antimonial compounds in pregnancy should generally be avoided due to an increased risk of miscarriage or preterm delivery, and there are no safety data on paromomycin in pregnancy.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
However, if no other options are available, treatment with these medications (alone or in combination) may be an option under expert guidance only.
Miltefosine must be avoided due to fetal risk.
Visceral leishmaniasis: treatment in immunocompromised patients
Immunocompromise among VL patients is most commonly due to HIV co-infection followed by iatrogenic immunosuppression (e.g., solid organ transplant recipients). Immunocompromising conditions not only increase the risk of developing symptomatic disease but also decrease the chance of initial cure while increasing the risk of relapse.[167]Akuffo H, Costa C, van Griensven J, et al. New insights into leishmaniasis in the immunosuppressed. PLoS Negl Trop Dis. 2018 May 10;12(5):e0006375.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5944929
http://www.ncbi.nlm.nih.gov/pubmed/29746470?tool=bestpractice.com
[168]Gajurel K, Dhakal R, Deresinski S. Leishmaniasis in solid organ and hematopoietic stem cell transplant recipients. Clin Transplant. 2017 Jan;31(1).
http://www.ncbi.nlm.nih.gov/pubmed/27801541?tool=bestpractice.com
A comprehensive approach of treating the parasite, managing immunosuppression, monitoring for relapse, and, potentially, administering secondary prophylaxis is essential.
HIV/AIDS
Lifelong antiretroviral therapy should be initiated or continued in all patients with HIV infection according to current local guidelines.
Liposomal amphotericin-B is generally regarded as the best option for patients with VL and HIV infection.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[169]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: Leishmaniasis. 2017 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/leishmaniasis
[170]World Health Organization. WHO technical report series 949: control of the leishmaniases - report of a meeting of the WHO Expert Committee on the control of leishmaniases. 2010 [internet publication].
https://www.who.int/publications/i/item/WHO-TRS-949
A higher total dose is used in patients who are immunocompromised. However, even with higher doses, treatment can be unsatisfactory and relapse is common. A published trial in East Africa showed only a 55% efficacy for liposomal amphotericin-B monotherapy among patients with a median CD4 count of 69 cells/microlitre.[171]Diro E, Blesson S, Edwards T, et al. A randomized trial of AmBisome monotherapy and AmBisome and miltefosine combination to treat visceral leishmaniasis in HIV co-infected patients in Ethiopia. PLoS Negl Trop Dis. 2019 Jan 17;13(1):e0006988.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6336227
http://www.ncbi.nlm.nih.gov/pubmed/30653490?tool=bestpractice.com
Combination therapy should be considered in this context. In the trial of liposomal amphotericin-B in East Africa discussed above, the second arm showed 88% efficacy with the combination of liposomal amphotericin-B and miltefosine among patients with a median CD4 count of 54 cells/microlitre.[171]Diro E, Blesson S, Edwards T, et al. A randomized trial of AmBisome monotherapy and AmBisome and miltefosine combination to treat visceral leishmaniasis in HIV co-infected patients in Ethiopia. PLoS Negl Trop Dis. 2019 Jan 17;13(1):e0006988.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6336227
http://www.ncbi.nlm.nih.gov/pubmed/30653490?tool=bestpractice.com
In India, the combination of miltefosine and liposomal amphotericin-B was associated with 96% efficacy compared to miltefosine monotherapy at 85%.[172]Burza S, Mahajan R, Kazmi S, et al. AmBisome monotherapy and combination AmBisome-Miltefosine therapy for the treatment of visceral leishmaniasis in patients coinfected with human immunodeficiency virus in India: a randomized open-label, parallel-Arm, phase 3 trial. Clin Infect Dis. 2022 Oct 12;75(8):1423-32.
https://www.doi.org/10.1093/cid/ciac127
http://www.ncbi.nlm.nih.gov/pubmed/35147680?tool=bestpractice.com
The combination of sodium stibogluconate and paromomycin, highly effective in non-HIV-co-infected East Africans, had only a 56% cure rate and probably should be avoided unless it is the only option.[163]Kimutai R, Musa AM, Njoroge S, et al. Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral leishmaniasis in eastern Africa: results from a pharmacovigilance programme. Clin Drug Investig. 2017 Mar;37(3):259-72.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5315726
http://www.ncbi.nlm.nih.gov/pubmed/28066878?tool=bestpractice.com
The World Health Organization (WHO) suggests liposomal amphotericin-B plus miltefosine over liposomal amphotericin-B monotherapy in people with HIV co-infection in East Africa and South East Asia.[62]World Health Organization. WHO guideline for the treatment of visceral leishmaniasis in HIV co-infected patients in East Africa and South-East Asia. June 2022 [internet publication].
https://www.who.int/publications/i/item/9789240048294
Pentavalent antimonial compounds are an alternative in patients with HIV infection; however, increased mortality has been observed when compared with amphotericin-B, and higher rates of severe adverse reactions have been observed.[173]Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013 May 2;7(5):e2195.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3642227
http://www.ncbi.nlm.nih.gov/pubmed/23658850?tool=bestpractice.com
[174]Laguna F, Lopez-Velez R, Pulido F, et al. Treatment of visceral leishmaniasis in HIV-infected patients: a randomized trial comparing meglumine antimoniate with amphotericin B. Spanish HIV-Leishmania Study Group. AIDS. 1999 Jun 18;13(9):1063-9.
http://www.ncbi.nlm.nih.gov/pubmed/10397536?tool=bestpractice.com
[175]Delgado J, Macías J, Pineda JA, et al. High frequency of serious side effects from meglumine antimoniate given without an upper limit dose for the treatment of visceral leishmaniasis in human immunodeficiency virus type-1-infected patients. Am J Trop Med Hyg. 1999 Nov;61(5):766-9.
http://www.ncbi.nlm.nih.gov/pubmed/10586909?tool=bestpractice.com
Secondary prophylaxis in HIV/AIDS
Secondary prophylaxis with anti-leishmanial drugs should be given to all patients with a CD4 count <200 to 350 cells/microlitre (the higher CD4 cut-off is recommended by the Pan American Health Organization) due to the high risk of relapse.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[145]Pan American Health Organization. Guideline for the treatment of Leishmaniasis in the Americas. Second edition. Sep 2022 [internet publication].
https://iris.paho.org/handle/10665.2/56120
[169]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: Leishmaniasis. 2017 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/leishmaniasis
[170]World Health Organization. WHO technical report series 949: control of the leishmaniases - report of a meeting of the WHO Expert Committee on the control of leishmaniases. 2010 [internet publication].
https://www.who.int/publications/i/item/WHO-TRS-949
There is no consensus on the best regimen, but guidelines do recommend various options.
A prospective study of pentamidine prophylaxis in East Africa had a 46% relapse rate at 1 year among those with CD4 counts <200 cells/microlitre compared with an historically observed relapse rate of 60% to 70% when prophylaxis was not used.[176]Diro E, Edwards T, Ritmeijer K, et al. Long term outcomes and prognostics of visceral leishmaniasis in HIV infected patients with use of pentamidine as secondary prophylaxis based on CD4 level: a prospective cohort study in Ethiopia. PLoS Negl Trop Dis. 2019 Feb 21;13(2):e0007132.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6400407
http://www.ncbi.nlm.nih.gov/pubmed/30789910?tool=bestpractice.com
It is unclear when secondary prophylaxis should end. Some guidelines suggest stopping after CD4 counts reach >200 to 350 cells/microlitre, whereas others recommend indefinite therapy as the risk for relapse continues. Regardless, careful monitoring for relapse is essential in all patients with HIV infection.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[169]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: Leishmaniasis. 2017 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/leishmaniasis
In East Africa, relapse rates have been shown to be high even among those with CD4 counts >200 cells/microlitre, with history of prior relapse being a significant predictor of future relapse or death.[176]Diro E, Edwards T, Ritmeijer K, et al. Long term outcomes and prognostics of visceral leishmaniasis in HIV infected patients with use of pentamidine as secondary prophylaxis based on CD4 level: a prospective cohort study in Ethiopia. PLoS Negl Trop Dis. 2019 Feb 21;13(2):e0007132.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6400407
http://www.ncbi.nlm.nih.gov/pubmed/30789910?tool=bestpractice.com
In East Africa, a 37% relapse rate was observed over a 2 year follow-up after 12-18 months of pentamidine prophylaxis, mainly in those with a low basilar CD4 count.[177]Diro E, Ritmeijer K, Boelaert M, et al. Long-term clinical outcomes in visceral leishmaniasis/human immunodeficiency virus-coinfected patients during and after pentamidine secondary prophylaxis in Ethiopia: a single-arm clinical trial. Clin Infect Dis. 2018 Jan 18;66(3):444-51.
https://www.doi.org/10.1093/cid/cix807
http://www.ncbi.nlm.nih.gov/pubmed/29020217?tool=bestpractice.com
Other forms of immunosuppression
Includes solid organ transplant patients, lymphatic or haematological malignancy, or treatment with immunosuppressants for other indications.
If patients are on immunosuppressants for other indications, consider decreasing the dose or stopping these medications, if possible, although this is based on expert opinion and not on strong data.[168]Gajurel K, Dhakal R, Deresinski S. Leishmaniasis in solid organ and hematopoietic stem cell transplant recipients. Clin Transplant. 2017 Jan;31(1).
http://www.ncbi.nlm.nih.gov/pubmed/27801541?tool=bestpractice.com
Treatment has been accomplished with multiple regimens, but liposomal amphotericin-B is recommended, particularly in solid organ transplant patients.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[145]Pan American Health Organization. Guideline for the treatment of Leishmaniasis in the Americas. Second edition. Sep 2022 [internet publication].
https://iris.paho.org/handle/10665.2/56120
While relapse is common, particularly among solid organ transplant patients (approximately 25%), data are not clear on the role of secondary prophylaxis. The IDSA/ASTMH guidelines recommend against secondary prophylaxis among those without a prior relapse, but in a small retrospective case-control study, risk of relapse decreased by 27% among those on secondary prophylaxis.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[178]Clemente W, Vidal E, Girão E, et al. Risk factors, clinical features and outcomes of visceral leishmaniasis in solid-organ transplant recipients: a retrospective multicenter case-control study. Clin Microbiol Infect. 2015 Jan;21(1):89-95.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)00008-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25636932?tool=bestpractice.com
Consider secondary prophylaxis on an individual basis, especially among patients with a history of prior relapse for whom the immunosuppressive condition is ongoing.
Among all immunosuppressed patients who relapse, treatment with liposomal amphotericin-B is reasonable, even among those who received this as primary therapy, because failure in this setting is thought to be immunological rather than a result of drug resistance. Alternatively, other options or combination therapies can be tried.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
Pregnant patients
Treatment is essential because untreated visceral leishmaniasis can be fatal to both mother and fetus.
Limited data suggest that liposomal amphotericin-B is a safe and effective treatment option.[8]Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016 Dec 15;63(12):1539-57.
https://academic.oup.com/cid/article/63/12/1539/2645617
http://www.ncbi.nlm.nih.gov/pubmed/27941143?tool=bestpractice.com
[119]Pagliano P, Carannante N, Rossi M, et al. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. J Antimicrob Chemother. 2005 Feb;55(2):229-33.
https://academic.oup.com/jac/article/55/2/229/856828?login=false
http://www.ncbi.nlm.nih.gov/pubmed/15649998?tool=bestpractice.com
The other forms of amphotericin may be used if liposomal amphotericin is unavailable.
Post-kala-azar dermal leishmaniasis
There are few controlled studies on the management of post-kala-azar dermal leishmaniasis. Mild-to-moderate post-kala-azar dermal leishmaniasis (PKDL) self-heals in the majority of patients of East African descent within 3 to 12 months. Treatment is indicated for East African patients with severe or non-self-healing PKDL, in Indian PKDL, and in patients who are immunocompromised.[6]Zijlstra EE, Musa AM, Khalil EA, et al. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003 Feb;3(2):87-98.
http://www.ncbi.nlm.nih.gov/pubmed/12560194?tool=bestpractice.com
In South Asia, miltefosine is the recommended first-line treatment for PKDL.[170]World Health Organization. WHO technical report series 949: control of the leishmaniases - report of a meeting of the WHO Expert Committee on the control of leishmaniases. 2010 [internet publication].
https://www.who.int/publications/i/item/WHO-TRS-949
A meta-analysis demonstrated concern for declining efficacy, which is being observed even at 12 weeks of therapy, and concerns for safety (e.g., ocular disorders including blindness, ulcerative keratitis, blurred vision, photophobia) with longer courses of treatment.[179]Pijpers J, den Boer ML, Essink DR, et al. The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia - a review and meta-analysis. PLoS Negl Trop Dis. 2019 Feb 11;13(2):e0007173.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6386412
http://www.ncbi.nlm.nih.gov/pubmed/30742620?tool=bestpractice.com
[180]World Health Organization. Statement on miltefosine - potential ocular disorders in patients treated with miltefosine for post-kala-azar dermal leishmaniasis (PKDL). Feb 2022 [internet publication].
https://www.who.int/news/item/10-02-2022-statement-on-miltefosine---potential-ocular-disorders-in-patients-treated-with-miltefosine-for-post-kala-azar-dermal-leishmaniasis-(pkdl)
Amphotericin-B is considered second-line in South Asia.[170]World Health Organization. WHO technical report series 949: control of the leishmaniases - report of a meeting of the WHO Expert Committee on the control of leishmaniases. 2010 [internet publication].
https://www.who.int/publications/i/item/WHO-TRS-949
Liposomal amphotericin-B has shown some efficacy in limited studies in the region.[181]Basher A, Maruf S, Nath P, et al. Case report: treatment of widespread nodular post kala-azar dermal leishmaniasis with extended-dose liposomal amphotericin B in Bangladesh – a series of four cases. Am J Trop Med Hyg. 2017 Oct;97(4):1111-5.
https://www.ajtmh.org/configurable/content/journals$002ftpmd$002f97$002f4$002farticle-p1111.xml?t:ac=journals%24002ftpmd%24002f97%24002f4%24002farticle-p1111.xml
http://www.ncbi.nlm.nih.gov/pubmed/28820697?tool=bestpractice.com
[182]Musa AM, Khalil EA, Mahgoub FA, et al. Efficacy of liposomal amphotericin B (AmBisome) in the treatment of persistent post-kala-azar dermal leishmaniasis (PKDL). Ann Trop Med Parasitol. 2005 Sep;99(6):563-9.
http://www.ncbi.nlm.nih.gov/pubmed/16156969?tool=bestpractice.com
However, molecular analysis demonstrated resurgence of parasites 6 months after treatment with liposomal amphotericin-B, but not with miltefosine.[183]Moulik S, Chaudhuri SJ, Sardar B, et al. Monitoring of parasite kinetics in Indian post-kala-azar dermal leishmaniasis. Clin Infect Dis. 2018 Jan 18;66(3):404-10.
https://academic.oup.com/cid/article/66/3/404/4157560
http://www.ncbi.nlm.nih.gov/pubmed/29020350?tool=bestpractice.com
In India, combination liposomal amphotericin-B and 45 days of miltefosine therapy showed 100% efficacy versus 90 days of miltefosine monotherapy (75% efficacy).[184]Ramesh V, Dixit KK, Sharma N, et al. Assessing the efficacy and safety of liposomal amphotericin B and miltefosine in combination for treatment of post kala-azar dermal leishmaniasis. J Infect Dis. 2020 Feb 3;221(4):608-17.
https://www.doi.org/10.1093/infdis/jiz486
http://www.ncbi.nlm.nih.gov/pubmed/31854451?tool=bestpractice.com
Pregnant patients
