Approach
Prominent risk factors for infection include exposure to sand fly bites, poor disease awareness, malnutrition, immunosuppression, and in some cases proximity to infected patients. History taking and physical examination are crucial to determine the degree of clinical suspicion for both cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL). Laboratory confirmation is mandatory, as other illnesses can present with a similar clinical picture.
History
In both VL and CL, the patient may present with a history of:
A previous stay in an endemic area [Figure caption and citation for the preceding image starts]: Status of endemicity of cutaneous leishmaniasis (CL) worldwide, 2020Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO (https://creativecommons.org/licenses/by-nc-sa/3.0/igo/) [Citation ends].
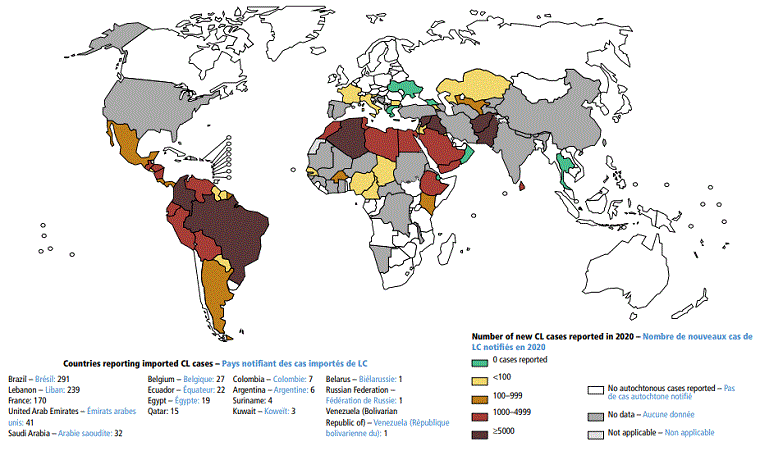 [Figure caption and citation for the preceding image starts]: Status of endemicity of visceral leishmaniasis (VL) worldwide, 2020Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO (https://creativecommons.org/licenses/by-nc-sa/3.0/igo/) [Citation ends].
[Figure caption and citation for the preceding image starts]: Status of endemicity of visceral leishmaniasis (VL) worldwide, 2020Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO (https://creativecommons.org/licenses/by-nc-sa/3.0/igo/) [Citation ends].
Cell-mediated-type immunosuppression, including the less mature immune system
Previous anti-leishmanial treatment (raises suspicion of relapse).
Presenting symptoms in VL include:
Prolonged fever
Fatigue
Anorexia
Weight loss
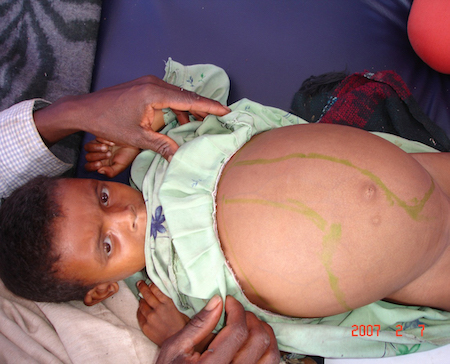
Abdominal distention. [Figure caption and citation for the preceding image starts]: Hepatosplenomegaly in an Ethiopian patient with visceral leishmaniasisImage courtesy of the World Health Organization [Citation ends].
Less common symptoms in VL include:
Cough
Diarrhoea
Rigors
Bleeding, including epistaxis.
Clinical examination
Presenting signs in CL include:
Ulcerative, nodular, plaque-like, or verrucous skin lesions at the bite site (localised CL). Lesions typically affect readily exposed skin, including the face, arms and lower limbs. A single ulcerative lesion involving the ear pinna is known as ‘chiclero's' ulcer in southeast Mexico and Latin America, when caused by Leishmania mexicana.[10]
Lesions at sites distant from the sand fly bite, such as areas of minor trauma.
Multiple non-ulcerative skin nodules (diffuse cutaneous leishmaniasis).
Many ulcerative and papulonodular skin lesions (disseminated leishmaniasis).
Nodulo-ulcerative lesions surrounding a healed leishmanial scar (leishmaniasis recidivans).
Small subcutaneous nodules (nodular lymphangitis) often in a sporotrichoid pattern, as well as regional lymphadenopathy in some cases.
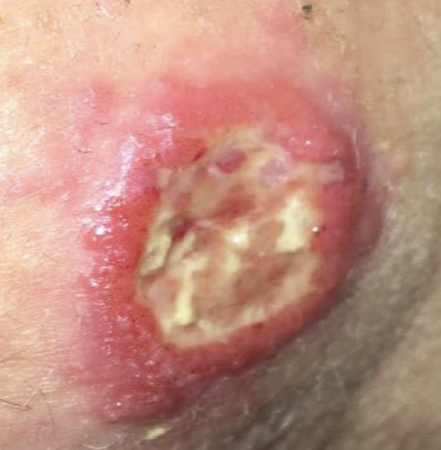
Granulomatous, ulcerating, and/or destructive mucosal inflammation most commonly in the nose, but may also involve the oropharynx or larynx (mucosal leishmaniasis [ML]). [Figure caption and citation for the preceding image starts]: Ulcerative Leishmania braziliensis lesion from a student who travelled to PeruFrom the collection of Dr N. Aronson; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Intralesional injection for the treatment of cutaneous leishmaniasisImage courtesy of the World Health Organization [Citation ends].
[Figure caption and citation for the preceding image starts]: Intralesional injection for the treatment of cutaneous leishmaniasisImage courtesy of the World Health Organization [Citation ends].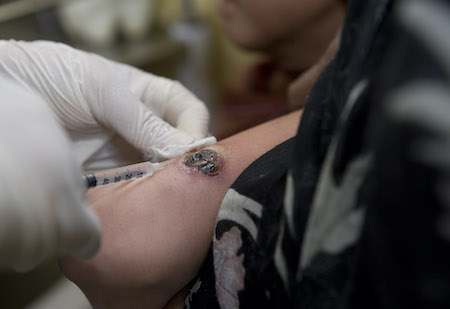
In VL, the following can be detected:
Wasting
Lymphadenopathy (common in Sudan, uncommon elsewhere)
Splenomegaly
Hepatomegaly
Hyperpigmentation (in South Asia).
In the majority of cases, the clinical presentation of VL in immunosuppressed patients is similar to that of immunocompetent patients. However, a minority of immunosuppressed patients present with atypical features (e.g., signs of digestive or respiratory tract involvement, lack of splenomegaly).
Investigations
A full blood count is recommended in VL to identify and monitor anaemia, leukopenia, and thrombocytopenia. Certain medications used in the treatment of leishmaniasis (e.g., paromomycin, pentavalent antimonial drugs, amphotericin-B, and miltefosine) may cause hepatic or renal dysfunction, necessitating measurement of liver function tests and urea at baseline. A human chorionic gonadotrophin (hCG) pregnancy test is essential prior to choosing treatment options as various medicines are toxic to the fetus. An ECG prior to using pentavalent antimonial drugs or pentamidine to assess the baseline QT interval is prudent.
Diagnosis is confirmed by microscopic examination of relevant specimens (histopathology), parasite isolation by blood or tissue culture, and molecular detection of parasite DNA by polymerase chain reaction (PCR); supplemental tests include serological testing in VL.[76][77][78] The choice of test depends on the type of leishmaniasis and test availability. Multiple tests are recommended, if possible, in order to maximise the likelihood of a positive result.[8]
Laboratory evaluation in cutaneous leishmaniasis
The skin lesion should be clean or debrided, if needed, prior to collection of a sample. If possible, multiple types of testing should be done to maximise the diagnostic yield.[79]
If available, molecular parasitological diagnosis (i.e., PCR-based assays) should be performed if CL is suspected. Such an approach is particularly useful in samples with a low parasite load (e.g., from patients with ML).[78] Molecular parasitological diagnosis is done using tissue samples obtained via skin scrapings (collecting tissue about the size of a rice grain), swabs of ulcers, aspirates, or slit smears. Skin punch or shave biopsies may actually have a lower yield as higher parasite burden is found in the epidermis and superior dermis.[80] Molecular methods are also used to determine the Leishmania species.
If molecular parasitological diagnosis is not available, use a microscopic examination of biopsy aspirates, smears, scrapings, or skin slit smears, staining with Giemsa or haematoxylin-eosin.[77] Microscopic examination is probably the most common diagnostic approach used in CL-endemic countries. A rod-shaped kinetoplast must be visualised.[1] The parasite is best seen using oil immersion microscopy, at a magnification of 100.
Parasite isolation by culture of tissue or biopsy samples may be useful, and is approximately 95% specific; however, this approach is not very sensitive (typically <50%), is labour intensive, and requires specialised laboratories. Species determination can be done biochemically (with isoenzyme electrophoresis), by mass spectroscopy (MALDI), or by using nucleic acid amplification. A knowledge of the Leishmania species responsible for infection helps direct therapy, especially when multiple species are circulating in a given area, and helps determine whether systemic treatment might be necessary (e.g., infection acquired in the Amazon region where the risk of ML is greater).[8]
Serology is not used in the diagnosis of CL due to poor sensitivity and an inability to distinguish between present and past infections.
Laboratory evaluation of visceral leishmaniasis in immunocompetent patients
VL can be suggested by one of several highly sensitive and specific serological tests (enzyme-linked immunosorbent assay [ELISA], indirect fluorescent antibody test, Western blot, direct agglutination test, rK39 antigen-based dipstick). The choice of test mainly depends on availability and laboratory expertise.[2] However, the sensitivity and specificity of these immunological assays may vary with geography, host immune response, and type of test. Furthermore, a positive result can persist for years after treatment.
In patients with a typical clinical presentation and positive VL serology, parasitological confirmation is advised, as the finding and quantification of parasites confirms the diagnosis and may help in assessing response to treatment. In patients with positive serology but atypical clinical disease, parasitological diagnosis is required. Parasitological specimens can be obtained by aspiration of splenic tissue, although this carries a risk of fatal haemorrhage; the bone marrow, which is the preferred method; the liver; or the lymph nodes. The sensitivity of microscopic examination of bone marrow or lymph node tissue can be <50%; therefore, a negative result does not rule out leishmaniasis infection. A sample of aspirate should be sent for culture and/or PCR, which are more sensitive than smear microscopy.[78]
In patients with negative serology, an alternative diagnosis should be sought.[2] However, if the clinical suspicion of VL remains high, parasitological tests are recommended.
Laboratory evaluation of visceral leishmaniasis in immunosuppressed patients
Parasitological diagnosis is the first-line approach in immunosuppressed patients, because serology is less sensitive in this cohort. Additionally, parasitological diagnosis is typically more sensitive because immunosuppressed patients have a higher parasite load in blood and tissues. Examination of peripheral blood smear or buffy coat by direct microscopy, culture, or PCR (the most sensitive method) is a non-invasive first-line test. If results are negative, the same procedures should be applied on a bone marrow aspirate. Depending on the clinical presentation, lesions in other body sites (e.g., digestive tract, skin) may be sampled.
Serological diagnosis is an adjunctive measure because of the variable, typically lower sensitivity of this testing method in immunosuppressed patients. Furthermore, a delay in the diagnosis and subsequent treatment has the potential to cause greater harm in this vulnerable patient group.[19][81]
Diagnosis of visceral leishmaniasis relapse
Patients with VL relapse usually have the same clinical presentation as the initial episode. Relapse should be parasitologically confirmed. Parasitological diagnosis by direct examination or tissue culture is preferred. The diagnostic value of PCR is uncertain, as PCR can remain positive in patients with clinical cure; however, quantitative PCR may show rising levels of DNA in relapse.[8][82] The diagnosis of relapse cannot be based on serological tests, as antibodies against Leishmania donovani or Leishmania infantum (synonym: Leishmania chagasi) usually remain detectable for years after initial diagnosis.[83][84]
Post-kala-azar dermal leishmaniasis
History-taking (present or past treatment of VL) and clinical examination (presence of macular, maculopapular, or nodular lesions at typical locations) are sufficient to initiate treatment for post-kala-azar dermal leishmaniasis. The diagnosis may be confirmed by direct examination, culture, or PCR of biopsies from skin lesions. The sensitivity of parasitological diagnosis is improved if large or nodular lesions are sampled.[6] Serological diagnosis is unhelpful, as specific antibodies against L donovani usually remain detectable for years after treatment of VL.[83][84]
Use of this content is subject to our disclaimer