The most common complication of priapism is complete erectile dysfunction (ED), which has been reported to occur in >50% of patients with priapism lasting 24-48 hours.[36]Zacharakis E, Raheem AA, Freeman A, et al. The efficacy of the T-shunt procedure and intracavernous tunneling (snake maneuver) for refractory ischemic priapism. J Urol. 2014 Jan;191(1):164-8.
http://www.ncbi.nlm.nih.gov/pubmed/23892191?tool=bestpractice.com
Therefore, the most critical factor in maintaining erectile function is immediate treatment of men presenting with priapism and prevention of future episodes. The primary goals of medical therapy for ischemic priapism are to relieve the pain and decompress the corporal bodies, thus reducing ischemia and the risk of tissue necrosis or injury.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Management depends on differentiating between ischemic, nonischemic, and stuttering forms of priapism. While ischemic priapism lasting >4 hours is a medical emergency and demands immediate treatment, episodes of ischemic or stuttering priapism lasting <4 hours should not be ignored. Delays in treatment and repetitive episodes of stuttering priapism lead to cellular, molecular, and morphologic changes in the corpus cavernosum, which over time result in tissue injury that places the patient at risk for the development of ED.[21]Bivalacqua TJ, Burnett AL. Priapism. In: Graham SD, Glen JF, eds. Glenn’s urologic surgery. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:487-91. Prompt treatment of all ischemic priapism episodes should be the goal.
Ischemic priapism
For ischemic priapism lasting up to 4 hours, observation or treatment are both acceptable options for management, depending on clinician or patient preference.[21]Bivalacqua TJ, Burnett AL. Priapism. In: Graham SD, Glen JF, eds. Glenn’s urologic surgery. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:487-91. However, delays in treatment predispose the patient to tissue injury that places the patient at risk for the development of ED. Therefore, prompt treatment of all episodes of ischemic or stuttering priapism are encouraged.
Ischemic priapism lasting >4 hours is an emergency and requires immediate treatment.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Management of ischemic priapism is approached in a stepwise fashion to achieve prompt resolution.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Medical management
Definitive first-line treatment consists of evacuation/aspiration of blood and irrigation of the corpora cavernosa, along with intracavernous injection of an alpha-adrenergic sympathomimetic agent.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
For anesthetic purposes, a preceding dorsal nerve block or local penile shaft block should be given.[5]Berger R, Billups K, Brock G, et al. Report of the American Foundation for Urologic Disease (AFUD) Thought Leader Panel for evaluation and treatment of priapism. Int J Impot Res. 2001 Dec;13(suppl 5):S39-43.
http://www.ncbi.nlm.nih.gov/pubmed/11781746?tool=bestpractice.com
Penile blood is aspirated using a nonheparinized syringe. Therapeutic aspiration may be performed simultaneously with cavernous blood gas sampling after insertion of a scalp vein needle (16 or 18 gauge) directly into the corpus cavernosum.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Concurrent irrigation with normal saline or a sympathomimetic agent to flush out blood from the cavernosum may be carried out.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Use of downward pressure on the glans of the erect penis also helps to evacuate blood out of the needles placed for irrigation and aspiration more readily. Careful attention should be made to not dislodge the needles with downward pressure and compression of the glans. If dislodged, additional needle sticks will be required for evacuation of blood.
Repeated aspirations or irrigations and sympathomimetic injections over several hours may be necessary and should be performed before initiating surgical intervention.[34]Burnett AL. Priapism. In: Wein AJ, Kavoussi LR, Novick AC, et al, eds. Campbell-Walsh urology. 9th ed. Philadelphia PA: Saunders Elsevier; 2007:839-49.
Many clinicians elect to perform intracavernosal injections without previous aspiration.
Phenylephrine is the preferred sympathomimetic agent because it has a lower risk of cardiovascular adverse effects than other agents and is associated with a higher rate of detumescence.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
However, if phenylephrine is unavailable, other alpha-adrenergic agonists may be used, such as ephedrine, epinephrine, norepinephrine, or metaraminol.
During and following intracavernous injection of any sympathomimetic, the patient should be monitored for known adverse effects (e.g., acute hypertension, headache, reflex bradycardia, tachycardia, palpitations, and cardiac arrhythmia). In all patients undergoing aspiration with irrigation, especially patients with high cardiovascular risk, BP and ECG monitoring are recommended.
If after a reasonable duration (some suggest 1 hour) and dose escalation of phenylephrine (some suggest 1000 micrograms of diluted phenylephrine over 1 hour) the penis is still tumesced, then a Doppler ultrasound should be considered to evaluate the status of the cavernosal arterial flow in the penis.[2]Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007 Nov;34(4):631-42.
http://www.ncbi.nlm.nih.gov/pubmed/17983902?tool=bestpractice.com
[5]Berger R, Billups K, Brock G, et al. Report of the American Foundation for Urologic Disease (AFUD) Thought Leader Panel for evaluation and treatment of priapism. Int J Impot Res. 2001 Dec;13(suppl 5):S39-43.
http://www.ncbi.nlm.nih.gov/pubmed/11781746?tool=bestpractice.com
Swelling and edema after appropriate detumescence with aspiration and irrigation may present like ischemic priapism, and therefore an ultrasound can rule in or out whether blood has been appropriately evacuated from the corpora cavernosa.
For priapism specifically related to sickle cell disease, medical therapies such as intravenous hydration, oxygenation, alkalinization, and exchange transfusion may be performed. However, these interventions should not precede the first-line treatment for all episodes of ischemic priapism: aspiration/irrigation in combination with an intracavernous alpha-agonist injection.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
[37]National Heart, Lung, and Blood Institute. Evidence-based management of sickle cell disease: expert panel report, 2014. Sep 2014 [internet publication].
https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease
[38]Mantadakis E, Ewalt DH, Cavender JD, et al. Outpatient penile aspiration and epinephrine irrigation for young patients with sickle cell anemia and prolonged priapism. Blood. 2000 Jan 1;95(1):78-82.
https://ashpublications.org/blood/article/95/1/78/180834/Outpatient-penile-aspiration-and-epinephrine
http://www.ncbi.nlm.nih.gov/pubmed/10607688?tool=bestpractice.com
US and European guidelines differ on the implementation of first-line therapies during ischemic priapism episodes of extended duration (>48 hours).[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
First-line treatments are unlikely to be successful in this circumstance and should be attempted at the surgeon's discretion.
Surgical shunt procedures
Ischemic or recurrent priapism refractory to medical therapy may require surgical intervention. Surgical shunts for ischemic priapism should be considered only if intracavernous injection of sympathomimetics has failed.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
The goal of surgery is to create a channel or fistula that allows the deoxygenated blood to drain from the corpora cavernosa.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
There are four subdivisions of shunts: percutaneous distal shunts, open distal shunts, open proximal shunts, and vein anastomoses/shunts.[39]Burnett AL. Surgical management of ischemic priapism. J Sex Med. 2012 Jan;9(1):114-20.
http://www.ncbi.nlm.nih.gov/pubmed/22221308?tool=bestpractice.com
Distal shunts should be performed first with proximal shunts potentially following if a distal shunt fails.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
A percutaneous distal corporoglanular shunt is the first choice, as it is simpler to perform and has lower complication rates than other approaches.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
In a Winter shunt procedure, a large-bore biopsy needle or biopsy gun is placed percutaneously through the glans penis.
If the previously mentioned percutaneous distal shunts are unsuccessful, an open distal shunt is the next step. The Al-Ghorab shunt involves excising a piece of the tunica albuginea from the tip of the corpus cavernosum. Modifications to Al-Ghorab corporoglanular shunt surgery have been described.[40]Burnett AL, Pierorazio PM. Corporal "snake" maneuver: corporoglanular shunt surgical modification for ischemic priapism. J Sex Med. 2009 Apr;6(4):1171-6.
http://www.ncbi.nlm.nih.gov/pubmed/19207268?tool=bestpractice.com
Consideration should be given to corporal tunneling (e.g., the Burnett snake maneuver) in patients with persistent ischemic priapism following distal corporoglanular shunt.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Distal shunts with corporal tunneling are associated with considerable success in relieving priapism, but may impinge upon post-procedure erectile function to a greater extent than distal shunting alone.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
When distal shunts fail, an open proximal shunt is the next line of treatment. However, proximal shunting is considered by some experts to be a historic procedure, having largely been replaced by distal shunts with tunneling procedures.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Proximal shunts such as the Quackels or Sacher shunt (creating a window between the corpus cavernosum and corpus spongiosum) may be considered.
Vein anastomoses/shunts should be performed as a last resort. The Grayhack shunt creates a window in the corpus cavernosum anastomosing the saphenous vein, whereas the Barry shunt creates a window in the corpus cavernosum anastomosing the deep dorsal vein.
For all shunt procedures, the patient should receive perioperative and postoperative antibiotics.[21]Bivalacqua TJ, Burnett AL. Priapism. In: Graham SD, Glen JF, eds. Glenn’s urologic surgery. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:487-91.[Figure caption and citation for the preceding image starts]: The technique of penile blood aspiration (corpora cavernosal needle placement for maximal corporal body irrigation)Arthur L. Burnett, MD, FACS [Citation ends].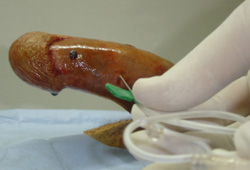 [Figure caption and citation for the preceding image starts]: Types of surgical shunt procedures for the treatment of ischemic priapismHelen R. Levey, DO, MPH [Citation ends].
[Figure caption and citation for the preceding image starts]: Types of surgical shunt procedures for the treatment of ischemic priapismHelen R. Levey, DO, MPH [Citation ends].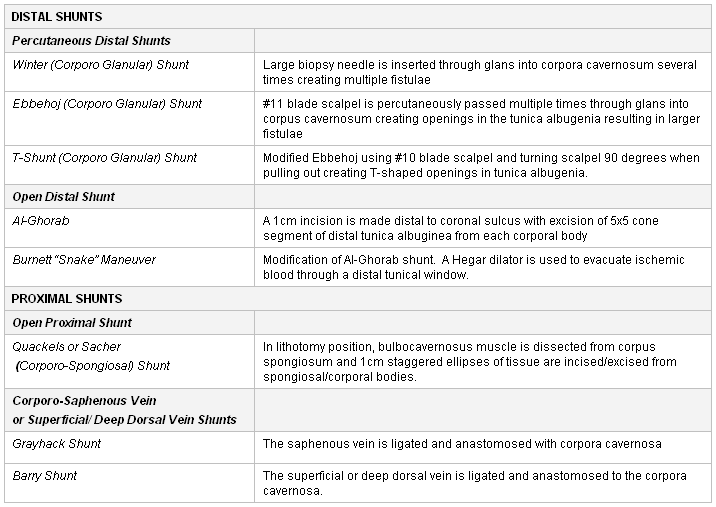
Penile prosthesis
US guidelines state that penile prosthesis placement can be considered for untreated acute ischemic priapism >36 hours or in those who are refractory to shunting, with or without tunneling.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
European guideline relative indications for penile prosthesis in patients with ischemic priapism include duration >48 hours, failure of medical management, MRI or corporal biopsy with evidence of smooth muscle necrosis, or failure of shunt procedures.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Penile implant placement should occur within 3 weeks after an episode of acute ischemic priapism, although patients who have undergone distal penile shunts may need to wait longer for proper healing of distal corporal tissue.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Penile implant should depend on the patient’s clinical scenario and the surgeon’s experience level. A malleable penile implant may offer less surgical and postoperative risk.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
For men who have presented with recurrent refractory episodes of ischemic priapism and have undergone surgical management for priapism, whether it was repeated aspiration and irrigation or more invasive repetitive shunt procedures, a penile prosthesis may be an appropriate alternative rather than subjecting the patient to additional future shunt procedures.[41]Ralph DJ, Garaffa G, Muneer A, et al. The immediate insertion of a penile prosthesis for acute ischaemic priapism. Eur Urol. 2009 Dec;56(6):1033-8.
http://www.ncbi.nlm.nih.gov/pubmed/18930579?tool=bestpractice.com
[42]Monga M, Broderick GA, Hellstrom WJ. Priapism in sickle cell disease: the case for early implantation of the penile prosthesis. Eur Urol. 1996;30(1):54-9.
http://www.ncbi.nlm.nih.gov/pubmed/8854068?tool=bestpractice.com
[43]Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology. 2006 May;67(5):1072-5.
http://www.ncbi.nlm.nih.gov/pubmed/16581112?tool=bestpractice.com
[44]Rees RW, Kalsi J, Minhas S, et al. The management of low-flow priapism with the immediate insertion of a penile prosthesis. BJU Int. 2002 Dec;90(9):893-7.
http://www.ncbi.nlm.nih.gov/pubmed/12460352?tool=bestpractice.com
[45]Tausch TJ, Evans LA, Morey AF. Immediate insertion of a semirigid penile prosthesis for refractory ischemic priapism. Mil Med. 2007 Nov;172(11):1211-2.
http://www.ncbi.nlm.nih.gov/pubmed/18062399?tool=bestpractice.com
Nonischemic priapism
The initial management of nonischemic priapism is observation with an option for conservative treatment.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Conservative approaches
US guidelines recommend 4 weeks as a reasonable observation period, unless the patient is experiencing significant discomfort. After the 4-week monitoring period, the fistula should be re-evaluated with color duplex ultrasonography to assess if it has started to close. If the fistula is unchanged, or if the patient is experiencing ongoing discomfort, intervention may be considered.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Conservative treatment methods include applying ice to the perineum or perineal compression, with or without ultrasound guidance.[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Spontaneous resolution is seen in the majority of cases, although erectile dysfunction of some form may occur in some patients.[46]Moussa M, Abou Chakra M, Papatsoris A, et al. An update on the management algorithms of priapism during the last decade. Arch Ital Urol Androl. 2022 Jun 30;94(2):237-47.
https://www.pagepressjournals.org/index.php/aiua/article/view/aiua.2022.2.237/10206
http://www.ncbi.nlm.nih.gov/pubmed/35775354?tool=bestpractice.com
Embolization
Invasive interventions can be performed at the patient's request, but the likelihood of spontaneous resolution, risks of treatment-related erectile dysfunction, and the relatively low risk of complications if no active treatment is performed should all be discussed with the patient before any procedure is performed.
Failure of observation or conservative management warrants discussion of treatment with selective arterial embolization.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
Both resorbable (i.e., autologous clot, gel foam) and nonresorbable (i.e., microcoils, polyvinyl alcohol [PVA] particles) embolization materials are available for use and achieve similar results.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Some studies suggest that autologous clot is associated with the highest recurrence rate, and that PVA particles provide the best recovery of erectile function; however, data remain inconclusive.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
[4]European Association of Urology. Guidelines: sexual and reproductive health. 2022 [internet publication].
https://uroweb.org/guidelines/sexual-and-reproductive-health#9
If an initial attempt at embolization fails, patients should be offered a second attempt, ideally with nonresorbable PVA particles.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Studies suggest that embolization leads to resolution of nonischemic priapism in 85% of patients, with 80% retaining functional erections. However, embolization carries a risk of erectile dysfunction; priapism may recur.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Surgery
Surgical management of nonischemic priapism should be considered only if attempts at repeat embolization have failed. Usually, this involves direct surgical ligation of cavernosal sinusoidal fistulae or pseudoaneurysms. Surgery should be performed with intraoperative color duplex ultrasonography.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Recurrent (stuttering) priapism
Treatment should focus on preventing future episodes; management of individual episodes should follow that for ischemic priapism.
Preventive strategies
Efficacy and safety data remain insufficient to recommend optimal preventive strategies with certainty.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Oral baclofen, dutasteride (a 5-alpha-reductase inhibitor), tadalafil or sildenafil (phosphodiesterase-5 inhibitors), ketoconazole with prednisone, pseudoephedrine, cyproterone (an antiandrogen), and aspirin have all been used with varying degrees of success. Etilefrine, hydroxyurea, and automated exchange transfusion may be considered in addition to these therapies in individuals with recurrent priapism and sickle cell disease (SCD).[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
One meta-analysis of different drug treatments, including sildenafil and ephedrine, found no significant effect attributable to any of the treatments, compared with placebo, in relation to reduction in frequency of stuttering priapism in patients with SCD.[47]Chinegwundoh FI, Smith S, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Syst Rev. 2020 Apr 6;(4):CD004198.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004198.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/32251534?tool=bestpractice.com
Ketoconazole with prednisone appears to be the most effective pharmacologic intervention for the prevention of recurrent (stuttering) ischemic priapism.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Expert guidance is required; ketoconazole may cause severe liver injury and adrenal insufficiency.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
Liver and adrenal function should be monitored before and during treatment.
Hormonal agents such as ketoconazole and cyproterone should not be used in patients who have not achieved full sexual maturation and adult stature.
Home self-injection
Intracavernosal self-injection of phenylephrine or other sympathomimetic (e.g., ephedrine, epinephrine, norepinephrine, or metaraminol) may be considered in patients refractory to or who reject systemic treatment; however, it is not a preventive strategy.[1]Bivalacqua TJ, Allen BK, Brock GB, et al. The diagnosis and management of recurrent ischemic priapism, priapism in sickle cell patients, and non-ischemic priapism: an AUA/SMSNA guideline. J Urol. 2022 Jul;208(1):43-52.
https://www.auajournals.org/doi/10.1097/JU.0000000000002767
http://www.ncbi.nlm.nih.gov/pubmed/35536142?tool=bestpractice.com
The patient should be counseled regarding the administration and adverse effects of sympathomimetic agents. If an episode of priapism occurs, the patient can perform the injection at home after being shown how to do so in the clinic. However, it is essential that he be instructed to seek medical attention if the priapism lasts >3 hours, because prompt medical treatment is necessary.
 [Figure caption and citation for the preceding image starts]: Types of surgical shunt procedures for the treatment of ischemic priapismHelen R. Levey, DO, MPH [Citation ends].
[Figure caption and citation for the preceding image starts]: Types of surgical shunt procedures for the treatment of ischemic priapismHelen R. Levey, DO, MPH [Citation ends].