Details
Prenatal care is a key component of a healthy pregnancy. Regular prenatal care helps to identify and treat complications and promote healthy behaviors. Outcome data suggest that babies born to mothers who do not receive prenatal care are three times more likely to be of low birth weight, and five times more likely to die, compared with babies born to mothers who receive prenatal care. US Department of Health and Human Services: prenatal care fact sheet Opens in new window In addition to medical care, prenatal care includes counseling and education.[1] This topic provides an overview for the prenatal management of healthy pregnant women with singleton pregnancies.
Ideally, prenatal care begins before conception. Preconception care has been defined as a set of interventions to identify and modify biomedical, behavioral, and psychosocial risks to a woman's health or pregnancy outcome through prevention and management. Preconception care should be considered not as a single visit, but as a continuum of care throughout a woman's reproductive life.[2] Because a significant proportion of pregnancies are unintended, the negative consequences of many behaviors, illnesses, and medications can affect fetal development early in pregnancy; often damage to the fetus occurs before a woman even realizes that she is pregnant. Thus, all routine healthcare encounters during a woman's reproductive years should include counseling on medical care and healthy behaviors to optimize pregnancy outcome.[2][3] For example, healthy women should begin folic acid supplementation (400-800 micrograms/day), ideally at least 3 months before conception and continue until at least 12 weeks' gestation.[2][3][4][5][6]
Factors affecting pregnancy outcome should include consideration of age, family history, genetic history, nutritional status, folic acid intake, environmental and occupational exposures, and teratogens. A history of illicit substance use, tobacco and alcohol consumption, medical conditions, medication, immunization status, risk factors for sexually transmitted infections (STIs), psychosocial concerns (depression, domestic violence), and pregnancy spacing is required.[3]
Women with diabetes or chronic hypertension should be counseled on optimizing glycemic or blood pressure control, and pregnancy should be discouraged until control is achieved. Referral to a specialist for advice on the risks and benefits of treatment should be offered.[7][8]
Women who are planning a pregnancy should be counseled regarding the relative risks and benefits of valproate use during pregnancy, and alternative therapeutic options should be considered. In the US, standard practice is that valproate and its analogs are only prescribed for the treatment of mental health problems in women who are planning a pregnancy, pregnant or considering breast-feeding if other alternative medications are not acceptable or not effective, or if the woman has responded only to valproate in the past.[9] In 2018, the European Medicines Agency recommended that valproate and its analogs are contraindicated during pregnancy because of the risk of congenital malformations and developmental problems in the infant/child.[10] In both Europe and the US, valproate and its analogs must not be used in women or girls of childbearing potential unless there is a pregnancy prevention program in place and certain conditions are met.[11][12]
Before becoming pregnant, women should have an up-to-date vaccination record. Women should avoid pregnancy for 1 month after receiving a live attenuated vaccine (e.g., rubella or varicella). CDC: guidelines for vaccinating pregnant women Opens in new window CDC: adult immunization schedule by age Opens in new window
Early and regular prenatal care is recommended to improve pregnancy outcomes. Recommendations include prenatal visits, nutritional care, education, and other patient-specific issues.[1]
Prenatal visits
Traditional prenatal care includes a series of between 7 and 11 visits; however, the number of visits necessary for adequate care is disputed. Though limited data are available regarding the optimal frequency, timing, and content of visits, the number of prenatal care visits should be determined according to the needs and risk status of each woman and her fetus.[1][13] In addition to scheduled routine visits, pregnant women should have access to unscheduled or emergency visits on a 24-hour basis.
Prenatal care visits should be scheduled at appropriate intervals to ensure time-sensitive testing and screenings, administration of Rho(D) immune globulin, if needed, and monitoring for common complications.
The typical frequency of visits in an uncomplicated pregnancy is as follows:
Every 4 weeks for the first 28 weeks
Every 2 to 3 weeks between 28 and 36 weeks
Weekly after 36 weeks.
More frequent visits may occur at the prenatal care provider's discretion or at a patient'’s request. Typical visits include evaluation of blood pressure, weight, testing urine for protein levels, and checking the fetal heart rate. Psychosocial screening at least once each trimester may help to identify issues that may require further evaluation, intervention, or outside referral.
In areas with limited resources, reduced-visit programs are associated with an increase in perinatal mortality compared with standard practice, although neonatal intensive care admissions may be reduced. Women also prefer the standard visits schedule. While fewer visits may be associated with lower costs, a standard visit schedule should be recommended to all patients.[14]
It is recommended that pregnant women initiate prenatal care by 10 to 12 weeks' gestation.[15] The first prenatal visit should include a comprehensive history, laboratory work, and education about pregnancy health. Height and weight should be recorded to calculate body mass index (BMI), which provides information to determine weight gain guidelines. [
 ]
At the first prenatal visit, all pregnant women should also have a blood test to check complete blood count, ABO blood group, Rh D status, and the presence of erythrocyte antibodies.[13][16]
]
At the first prenatal visit, all pregnant women should also have a blood test to check complete blood count, ABO blood group, Rh D status, and the presence of erythrocyte antibodies.[13][16]
Screening for depression, which is common during pregnancy and in the first 12 months after delivery, may be beneficial, particularly in women with a history of major depression. This enables the recognition of patients that may benefit from targeted therapy. Undiagnosed and untreated psychiatric illness is a risk to both the mother and fetus.[17][18] See Postpartum depression.
During the first appointment, ensure there is time for a private, one-to-one discussion with the women and ask sensitively about potential exposure to domestic abuse (assaultive and/or coercive behavior).[13][19][20] Women in the military or women veterans have a significantly increased risk of exposure to interpersonal violence, including sexual assault or abuse, and intimate partner violence.[21]
A comprehensive physical exam should be performed at the first or second visit. An initial exam will identify women with female genital mutilation/cutting (FGM/C). Because FGM/C may adversely affect birth outcomes and increase risk for obstetric complications, these patients may have special intrapartum care needs.[22]
At the second visit, a review of laboratory results will promote further discussion of a management plan.
Women who are due to give birth at age 35 years or older should be advised of the potential adverse outcomes for mother and baby, and counseled accordingly, and those due to give birth ages 40 years or older should be offered antepartum fetal surveillance because of the increased risk of stillbirth.[23]
If the woman has had a prior cesarean delivery, the risks and benefits of a trial of labor versus repeat cesarean delivery should be reviewed.[24] Candidacy for a trial of labor after cesarean should also be considered.
The American College of Obstetricians and Gynecologists (ACOG) and the Centers for Disease Control and Prevention (CDC) recommend that adults receive an annual influenza vaccine and women who are or will be pregnant through the influenza season (October through May) undergo (inactivated or recombinant) influenza vaccination as soon as it is available.[25] Those women who are pregnant in the respiratory syncytial virus (RSV) season (September through January) are also recommended to have a single dose of the maternal RSV vaccine between 32 and 36 weeks of gestation, to protect infants ages <6 months against RSV-associated lower respiratory tract infections.[26][27] ACOG and the CDC additionally recommend tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination for pregnant women, as early as possible between 27 and 36 weeks of gestation to maximize passive antibody transfer to the baby.[28] CDC: adult immunization schedule by age Opens in new window
US and UK guidelines recommend that all eligible individuals >6 months of age, including pregnant and lactating women, should be vaccinated against COVID-19.[29][30][31][32][33][34] CDC: adult immunization schedule by age Opens in new window
Appropriate fetal growth can be screened by measuring fundal height (symphysis to uterine fundus) from 24 to 38 weeks' gestation.[35] Fundal height in centimeters is approximately equal to the gestational age in weeks. Discrepancies of >3 cm should prompt ultrasound evaluation of amniotic fluid index and fetal growth.[36] The prenatal care provider should keep in mind that fundal height measurements can be influenced by numerous factors, including maternal size, bladder filling, uterine fibroids, multiple gestations, and fetal presentation.[35][37]
Mothers are questioned regarding pain, fetal movement, contraction frequency, vaginal bleeding, loss of fluid or discharge, other symptoms of preterm labor, and preeclampsia symptoms at appropriate gestation intervals, in addition to any other patient-provided complaints or concerns.[13]
Structured records assist in ensuring comprehensive, evidence-based care.[38]
Nutritional counseling
Nutrition education should focus on a well-balanced, varied, nutritional food plan consistent with the patient's food preferences. [
 ]
Obstetric care providers should calculate a woman’s BMI at the first prenatal visit. Using BMI as a guideline, appropriate weight gain should be discussed.[39][40][41][Figure caption and citation for the preceding image starts]: Weight gain during pregnancyAdapted from Institute of Medicine. Weight gain during pregnancy: re-examining the guidelines. 2009 [Citation ends].
]
Obstetric care providers should calculate a woman’s BMI at the first prenatal visit. Using BMI as a guideline, appropriate weight gain should be discussed.[39][40][41][Figure caption and citation for the preceding image starts]: Weight gain during pregnancyAdapted from Institute of Medicine. Weight gain during pregnancy: re-examining the guidelines. 2009 [Citation ends].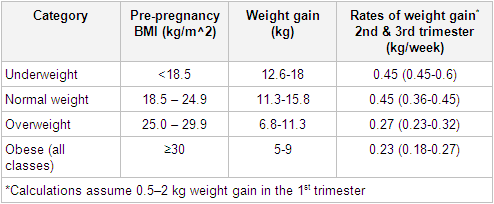 [
[  ]
]
A nutrition consultation may be offered to obese pregnant women. Obese women should be counseled on healthy eating, and encouraged to participate in physical activity which will support weight management during and after pregnancy.[42][43][44]
Weight gain and caloric intake recommendations are higher for women pregnant with twins or higher-order multiple gestations. The daily recommended caloric intake for women with a normal BMI, who are pregnant with twins, is 40-45 kcal/kg daily (18-20 kcal/lb).[45] Women with multiple gestations have a higher incidence of anemia, compared with those who have singleton pregnancies, and so a blood test should be performed at 20 to 24 weeks to determine whether they need early supplementation with iron or folic acid.[46] Additional calcium, magnesium, and zinc supplementation may also be required for patients carrying multiple gestations over routine prenatal vitamin intake.
Gestational weight gain greater than or less than the Institute of Medicine guidelines appears to be associated with higher risk of some adverse maternal and newborn outcomes.[47]
Diet and physical activity-based interventions in pregnancy are beneficial and can reduce gestational weight gain, as well as the rate of cesarean delivery.[48]
The Food and Drug Administration has made specific recommendations about fish intake for women of childbearing age. FDA: eating fish - what pregnant women and parents should know Opens in new window
Listeriosis is a bacterial illness that can be particularly harmful to pregnant women, possibly resulting in miscarriage or stillbirth. To prevent listeriosis, pregnant women should avoid unpasteurized milk, soft cheeses, raw sprouts, some types of cold smoked fish, and some types of pâté.[3][49] Additionally, pregnant women can reduce their risk of salmonella infection by avoiding raw or partially cooked eggs or food that may contain them (e.g., mayonnaise) and raw or partially cooked meat, especially poultry.
Daily prenatal vitamins containing folate (400-800 micrograms/day) are recommended as tolerated throughout pregnancy, and at least through the first 3 months of pregnancy.[3] Ideally, women should start folate supplementation 12 weeks prior to conception.[6] Selected presentations, such as a prior pregnancy complicated by fetal neural tube defect, require higher-dose folate intake of 4 mg/day.[50] When started preconceptually and continued through pregnancy, folic acid supplementation may reduce risk of small-for-gestational-age neonates at birth.[51]
Preterm birth is associated with lower levels of omega-3 fatty acid docosahexaeonoic acid (DHA), which has an important role in neurodevelopment. While observational data suggest that DHA supplementation has a beneficial effect on pediatric neurodevelopmental outcomes, randomized controlled trials have yielded conflicting results.[52][53] There is insufficient evidence to support DHA supplementation to prevent preterm birth.[54] There is also no evidence that such supplementation reduces the risk of preeclampsia or gestational diabetes mellitus.[55]
Vitamin C and E supplementation during pregnancy does not prevent preeclampsia.[56] While it may potentially have some benefit for the prevention of placental abruption and preterm premature rupture of membranes (PPROM), vitamin C alone or in combination with other supplements is unproven to reduce risk of fetal or neonatal death, poor fetal growth, preeclampsia, or preterm birth.[57] [
 ]
]
Moderate caffeine intake does not seem to have negative effects on pregnancy; however, caffeine intake should be limited to <200 mg daily.[58]
Alcohol consumption is contraindicated in pregnancy. In the UK, Department of Health guidelines recommend that for pregnant women and women planning a pregnancy, the safest approach is not to drink alcohol at all, to keep risks to the baby to a minimum.[59] In the US there are no accepted guidelines regarding an acceptable alcohol intake in pregnancy. Prenatal drinking poses potentially serious consequences to both mother and fetus. Brief standardized screening questionnaires (Tolerance, Annoyance, Cut down, Eye opener [T-ACE], Alcohol Use Disorders Identification Test [AUDIT-C], Tolerance, Worried, Eye-opener, Amnesia, Cut down [TWEAK]) show promise as screening tools to identify risk drinking in pregnant women, although further investigation is required.[60]
Education
Education to promote maternal and fetal health and safety is a significant component of prenatal care.
At every prenatal appointment the women should be asked about their general health and wellbeing and healthy behaviors should be promoted, but also consider these specific issues:[13][15]
Smoking: if women use tobacco products, smoking cessation should be encouraged and support provided.[61][62] [
 ]
[
]
[  ]
[
]
[  ]
]
Substance misuse: women who regularly misuse recreational drugs, over-the-counter medications, prescription medications, volatile substances (such as solvents or inhalants) to an extent whereby physical dependence or harm is a risk to themselves and/or their unborn baby should be identified and treated.[63]
Working: women with an uncomplicated pregnancy can typically continue working until the onset of labor. Women with medical complications or other pregnancy complications, may need to make adjustments.
Air travel: women with uncomplicated pregnancies can fly safely until 36 weeks' gestation. ACOG: travel during pregnancy Opens in new window Pregnant women who are planning to fly should be informed about the increased risks of venous thromboembolism from the combination of pregnancy and venous stasis, and instructed to take appropriate precautions (support stockings, movement of lower extremities, hydration).[64]
Exercise: women should be encouraged to continue or begin a moderate aerobic exercise program during pregnancy.[65][66] Although the limited available randomized controlled trial data do not clearly support a benefit of exercise during pregnancy for the prevention of glucose intolerance or gestational diabetes mellitus, there may be other physical and psychological benefits derived from aerobic exercise in pregnancy.[67] [
 ]
Structured physical exercise has been demonstrated to significantly reduce the risk of delivering a macrosomic or large-for-gestational-age newborn without influencing the risk of having a small newborn.[68] When not otherwise contraindicated, physical exercise during pregnancy may reduce risk of cesarean delivery.[69] Potential risks from contact sports, high-impact sports, activities with risk of abdominal trauma, and scuba diving should also be discussed.
]
Structured physical exercise has been demonstrated to significantly reduce the risk of delivering a macrosomic or large-for-gestational-age newborn without influencing the risk of having a small newborn.[68] When not otherwise contraindicated, physical exercise during pregnancy may reduce risk of cesarean delivery.[69] Potential risks from contact sports, high-impact sports, activities with risk of abdominal trauma, and scuba diving should also be discussed. Childbirth education: attendance in childbirth education classes may be considered. Classes teach expectant mothers about the relative risks and benefits of home versus hospital birth, labor and delivery, pain relief options, potential obstetric complications and procedures, normal newborn care, and postpartum adjustment.[70]
Breast-feeding: throughout prenatal care, healthcare providers should provide information about the benefits of breast-feeding and breast-feeding support should be provided.[71]
Miscellaneous: other educational issues to discuss during the antepartum period include dental care, nutrition, wearing a seat belt, minimal use of hot tubs or saunas, hazardous-chemical exposure, sleep position, sexual activity, postpartum contraception, and circumcision of male infants.
All women require a complete blood count (CBC), ABO blood group, Rhesus status, presence of erythrocyte antibodies, rubella status, hepatitis B screening, screening for STIs (syphilis, HIV, gonorrhea, and chlamydia), group B streptococcus (GBS) screening, urine dipstick and culture, blood pressure measurement, gestational diabetes screening, check of last cervical screening, and ultrasound.[13][16][72][73][74][75][76][77][78][79] Other investigations may be performed as indicated.
[Figure caption and citation for the preceding image starts]: Recommended routine testingFrom the collection of Dr L.M. Szymanski and Dr J.L. Bienstock, amended by Dr M.E. D'Alton and Dr R.S. Miller [Citation ends].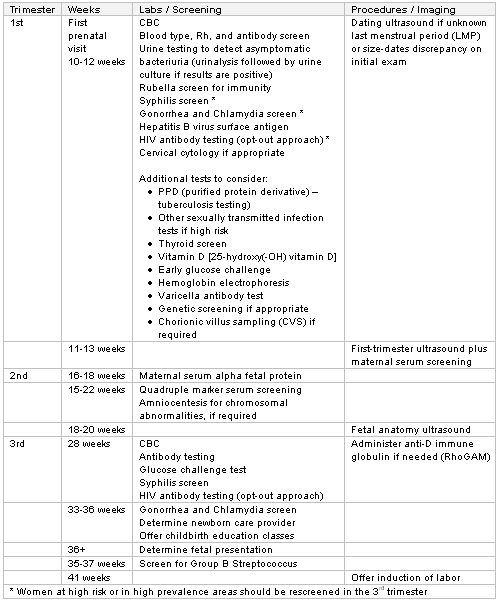
Routine screening for bacterial vaginosis in asymptomatic women and women not at risk for preterm delivery is not recommended.[80][81][82]
Public health departments in certain states may require additional testing (e.g., more frequent syphilis screening).[82] In addition to routine laboratory tests, several other screening tests should also be considered, many of which are based on racial and ethnic background or family history.
STIs
Women living in areas with high rates of STIs, or those with previous antepartum STI should be considered for repeat STI testing in the third trimester.[82] All women should be screened for syphilis at the first prenatal care visit, followed by universal rescreening during the third trimester and at birth.[79]
Vitamin D
Routine screening of all pregnant women for vitamin D deficiency is not recommended; only those at high risk, including vegetarians, women with dark skin, those who live in northern latitudes, and those whose clothes leave little skin exposed, should be tested during pregnancy.[83][84] Most women can ensure they receive enough vitamin D by taking prenatal supplements. If deficiency of vitamin D is identified during pregnancy, supplementation with 1000-2000 units/day of vitamin D is considered to be safe.[83] The Institute of Medicine has published a report on the dietary reference intakes for calcium and vitamin D advising an Estimated Average Requirement (EAR) for vitamin D of 400 IU/day (10 micrograms/day) with a Recommended Daily Allowance (RDA) of 600 IU/day (15 micrograms/day) during pregnancy.[85] Additionally, in the UK, updated UK-based National Institute for Health and Care Excellence (NICE) guidelines recommend vitamin D supplementation (10 micrograms/day) during pregnancy and while breastfeeding.[86]
Thyroid function tests
Universal screening for subclinical hypothyroidism is controversial. Although several major societies favor routine screening in women who are pregnant or planning pregnancy, ACOG maintains that data are insufficient to warrant routine screening and continues to recommend testing in symptomatic women and those with a personal or family history of thyroid disease or other medical conditions associated with thyroid disease, such as type 1 diabetes.[87] [
 ]
[
]
[  ]
To date, evidence does not support treatment of subclinical hypothyroidism as an intervention to improve pregnancy outcomes or neurocognitive function in neonates.[87][88][89]
]
To date, evidence does not support treatment of subclinical hypothyroidism as an intervention to improve pregnancy outcomes or neurocognitive function in neonates.[87][88][89]
Carrier screening
Ideally, genetic counseling should be performed before carrier screening.[90] ACOG recommends that all patients who are considering pregnancy or already pregnant are offered screening/tests for:[91]
Cystic fibrosis
Spinal muscular atrophy
Complete blood count and screening for thalassemias and hemoglobinopathies.
Hemoglobin electrophoresis should be performed in addition to a complete blood count if hemoglobinopathy is suspected based on African, Mediterranean, Middle Eastern, Southeast Asian, or West Indian ethnicity.[90]
In the US, ACOG recommends universal testing for hemoglobinopathy traits in people planning pregnancy or at the first prenatal visit if no previous results are available.[92]
Supplemental ethnic-based screening strategies also include:[90][91]
Tay-Sachs disease (Ashkenazi Jews, French Canadians, Cajuns)
Screening panels that detect mutations associated with disorders that commonly occur in Ashkenazi Jewish populations: Bloom syndrome, Canavan disease, cystic fibrosis, familial dysautonomia, Fanconi anemia group C, Gaucher disease, glycogen storage disease type 1a, maple syrup urine disease types 1A and 1B, mucolipidosis IV, Niemann-Pick disease type A, and Tay-Sachs disease.
Additional testing can be considered depending upon familial history, or on patient and physician preference:
Fragile X syndrome for women with a family history of fragile X-related disorders or intellectual disability suggestive of fragile X syndrome, or women with a personal history of ovarian insufficiency; additional screening may be indicated based on family history or ancestry[91]
Hemochromatosis for people of Celtic ancestry (especially if a positive family history exists)
Duchenne muscular dystrophy.
Expanded carrier screening represents an alternative approach in which a large number of conditions are screened for simultaneously, and modern products can screen for as many as several hundred conditions. This approach is gaining in popularity due to its efficiency and cost value.[91]
First-trimester ultrasound screening is optimal for pregnancy dating; however, a complete anatomic evaluation cannot be performed at this time. In the UK, NICE recommends women are offered a first-trimester ultrasound between 11+2 weeks and 14+1 weeks' gestation.[13] In the US, however, ACOG recommends the first-trimester ultrasound is performed before 14+0 weeks' gestation.[93][94] Advantages of first-trimester ultrasonography include its ability to help determine the presence of an intrauterine pregnancy, gestational age, and evaluate for multiple gestation.[95] If a size-dates discrepancy is present upon initial ultrasound exam, or if menstrual dates are uncertain, a second- or third-trimester ultrasound exam is indicated for dating using a composite of fetal biometric measurements.[93][94][96]
An ultrasound performed as part of combined first-trimester aneuploidy screening will also provide accurate dating information.[94]
An ultrasound examination performed in the second-trimester is optimal for a survey of the fetal anatomy and placental location. In the UK, NICE recommends women are offered an ultrasound scan to take place between 18+0 and 20+6 weeks' gestation.[13] In the US, however, ACOG recommends the second-trimester ultrasound is performed between 18 and 22 weeks' gestation.[94] Second-trimester ultrasound examination can also confirm pregnancy dating if an earlier ultrasound was not performed. Any pregnancy without ultrasound examination confirming or revising the estimated due date before 22+0 weeks' gestation is considered suboptimally dated.[93][97]
The sensitivity of ultrasound to detect fetal anomalies ranges from 13% to 82%, depending on the type of anomaly, the level of risk in the population, and the expertise of the operators.[98] Additional ultrasound examinations are unnecessary unless maternal or fetal indications are present, although a common practice for incomplete anatomic surveys includes an interval follow-up ultrasound study.[96][99]
Women should be informed of the limitations of routine ultrasound.[15][94][100] Ultrasonography in pregnancy is not associated with adverse maternal, perinatal, or childhood outcomes, but the As Low As Reasonably Achievable (ALARA) principle recommends expose of women and babies to the least amount of ultrasound energy needed to obtain diagnostic information.[101][102] Long-term follow-up data from a randomized, controlled trial found no significant effect of second-trimester ultrasound exposure on overall school performance in teenagers.[103]
Obese patients should be informed that their fetus is at increased risk for congenital anomalies, including undiagnosed anomalies.[42][43] Sonographic fetal anatomic assessment may be better performed at 20 to 22 weeks in obese patients.[104]
Routine second trimester ultrasound examination will reveal placental location and relationship to the internal cervical os. Placenta previa complicates 0.3% to 0.5% of pregnancies.[105][106][107] The majority (>80%) of previa cases diagnosed at 20 weeks' gestation resolve by term.[108] Thus, a follow-up ultrasound in the third trimester, usually around 32 weeks' gestation, is warranted.[94][109][110] In women with a persistent low-lying placenta or placenta previa at 32 weeks' gestation, a further ultrasound should be arranged at 36 weeks to aid delivery planning.[111][112]
See Placenta previa.
Aneuploidy
Defined as an abnormal number of chromosomes, with 46 chromosomes representing normal in humans.
There may be fewer chromosomes, as in Turner syndrome (monosomy X), or more chromosomes, as in Down syndrome (trisomy 21) or other trisomies.
Chromosomal abnormalities occur in approximately 1:150 live births, with trisomy 21 (Down syndrome), trisomy 18, and trisomy 13 accounting for the majority.[113] Trisomy 18 and trisomy 13 are often incompatible with survival.[114]
Although the frequency of aneuploidies increases with advancing maternal age, they can present at any age (most children with Down syndrome are actually born to women younger than 35 years of age).[23][115] The ACOG recommends that all women, regardless of age, who present for prenatal care should be offered aneuploidy screening.[115] Women should be counseled on the age-related risk of fetal aneuploidy, the implications of a diagnosis, and all available screening tests, including their advantages and disadvantages.[23] In addition, all women should have the option of invasive prenatal testing (chorionic villus sampling [CVS] or amniocentesis) for diagnosis of fetal aneuploidy and other genetic abnormalities.[115] [
 ]
[
]
[  ]
]
Screening tests for aneuploidy
Prenatal genetic screening tests are available to all pregnant women, regardless of age or risk, and include serum screening with or without nuchal translucency ultrasound and cell-free DNA screening. Women should choose one of these prenatal genetic screening approaches, rather than multiple screening approaches performed simultaneously.
First-trimester screening can generally be performed from 10 to 14 weeks' gestation (when the crown-rump length measures between 38 and 45 mm and 84 mm) and includes ultrasound evaluation for nuchal translucency thickness plus maternal serum screening (pregnancy-associated plasma protein A, human chorionic gonadotropin, alpha-fetoprotein).[115] The detection rate for Down syndrome, at a positive screening rate of 5%, ranges from 82% to 90%.[114][115][116] Adding evaluation of the nasal bone, which is absent in 60% to 70% of fetuses with Down syndrome, can increase the detection rate to 95%. Technical standards and guidelines are available.[117]
Second-trimester screening can generally be performed from 15 to 23 weeks' gestation and includes a maternal quadruple marker screening ("quad screen"; human chorionic gonadotropin, alpha-fetoprotein, estriol, and inhibin), with a detection rate for Down syndrome of 80%.[114][115]
Combining screening results from both first- and second-trimester screening tests (i.e., integrated and sequential screening) can improve aneuploidy detection rates.[114][115]
It is important to be aware that when maternal serum is screened for the usual clinical indications (i.e., aneuploidy and neural tube defect), abnormal analyte results may also identify pregnancies at risk for adverse outcomes, such as preeclampsia, and these women should be referred for appropriate follow-up or consultation.[118]
Noninvasive prenatal aneuploidy screening for trisomies 21, 18, and 13 using cell-free fetal DNA is an option that has more recently become available to women with pregnancies at increased risk of aneuploidy. Cell-free fetal DNA screening has high sensitivity and specificity for trisomies 18 and 21, and lower sensitivities for trisomy 13 and sex chromosome abnormalities.[115] Patients should be informed that cell-free fetal DNA is not a substitute for definitive CVS or amniocentesis results, and it is limited in its ability to detect all chromosomal abnormalities and other genetic conditions.
Cell-free fetal DNA may be offered to appropriately counseled patients regardless of risk status.[115]
Cell-free DNA screening is not a diagnostic test: negative results cannot entirely exclude the possibility for aneuploidy and positive results should be confirmed with invasive genetic testing.[115]
All pregnant women should be offered an ultrasound in the second-trimester, which can be used to detect fetal anatomic markers associated with aneuploidy that may modify a pregnancy’s risk of carrying an affected fetus.[115] Examples of these sonographic markers include an echogenic intracardiac focus, absent nasal bone, short humerus or femur, and echogenic bowel.[94][114][115]
Whichever screening test is used, the patient should be advised that screening provides an individual risk assessment, but it is not diagnostic. Definitive genetic diagnosis requires invasive genetic testing.
Patients who desire definitive genetic information regarding their pregnancies should be counseled to consider invasive genetic testing. If interested, these patients should be referred to genetic counselors for further discussion.
Invasive testing[119]
Invasive testing must be performed to definitively diagnose chromosomal abnormalities.[120] In addition to conventional karyotype determination, comparative genomic hybridization microarray study is recommended in pregnancies complicated by a fetal anomaly.[121][122] Among women with structurally normal fetuses undergoing invasive diagnostic testing, either karyotype or chromosomal microarray analysis can be performed, and in many practices both are performed.
All women with a positive screening test, a risk result they are uncomfortable with, or an interest in invasive genetic testing should be offered genetic counseling and diagnostic testing.
Options for invasive genetic testing include CVS (at 10-13 weeks) and diagnostic amniocentesis (at ≥15 weeks). Patient counseling requires a discussion regarding the risks and benefits of each procedure, and a comparison of the relative merits and disadvantages between procedures. [
 ]
[
]
[  ]
]
Any discussion of invasive testing options must include disclosure that the procedure may cause obstetrical complications such as pregnancy loss or preterm rupture of membranes.
Some women may choose to bypass screening and proceed directly to diagnostic testing.[115] The option of invasive genetic testing should be available to all patients regardless of age or prior aneuploidy risk level.
See Turner syndrome and Down syndrome.
There are two approaches for NTD screening:
Maternal serum AFP (MS-AFP) in the second trimester (optimal at 15 to 18 weeks)
Ultrasound (optimal at 18 to 22 weeks).
MS-AFP has historically been used as a primary prenatal screening test: the standard screening cutoff, of 2.5 multiples of the median, detects between 65% and 80% of cases of open spina bifida and >95% of cases of anencephaly. If women screen positive, a detailed ultrasound is performed:
To ensure that the pregnancy is viable and accurately dated
To evaluate for the presence of multiple gestations
To assess fetal anatomy for NTDs and other defects associated with an elevated MS-AFP (omphalocele, gastroschisis, cystic hygroma).[50]
Amniocentesis can be performed to determine if amniotic fluid AFP is elevated and acetylcholinesterase is present, which are considered diagnostic of a NTD.[50][123][124]
Ultrasound has begun to supersede MS-AFP as a screening tool although these two screening modalities are not mutually exclusive. With experienced sonographers, the detection rate of NTDs approaches 95% to 100%.[123][124]
Most pregnant women with hypertensive disorders of pregnancy (gestational hypertension, preeclampsia or hemolysis, elevated liver enzymes, and low platelets [HELLP] syndrome) are asymptomatic, so all pregnant women should be screened by having their blood pressure (BP) measured and testing for the presence of protein in their urine at every routine prenatal appointment.[8][13][125][126][127] If the BP reading is >140/90 mmHg, a follow-up measurement must be obtained. At least two measurements should be taken, at least 4 hours apart.[126] If the urine dipstick test is positive, proteinuria should be quantified using spot testing, where available. If high BP persists, there should be a step up in care to an assessment in secondary care or admission to hospital, depending on clinical findings and symptoms.[8]
In the UK, NICE advocate screening for preeclampsia by identifying women with risk factors for the disease at the first prenatal appointment and again in the second trimester.[8][13] Prediction algorithms that use a combination of biomarkers and maternal history have been investigated to establish their potential as first-trimester screening tools for preeclampsia.[128][129] NICE recommends placental growth factor (PLGF)-based tests, used with standard clinical assessment, to help rule in or out preeclampsia for women with suspected presentation between 20 weeks and 36 weeks and 6 days of pregnancy.[130] The Federation of Gynecology and Obstetrics (FIGO) recommends that all women should be screened for preterm preeclampsia using a first‐trimester combined test as a one‐step procedure.[131] They suggest that the optimal test includes maternal risk factors, measurements of mean arterial pressure (MAP), serum PLGF, and uterine artery pulsatility index (UTPI). If it is not possible to measure PLGF and/or UTPI, the baseline screening test should be a combination of maternal risk factors with MAP, and not maternal risk factors alone. An algorithm derived from these factors, with the option to include serum pregnancy‐associated plasma protein A (PAPP‐A) is now available. FMF: risk for preeclampsia assessment Opens in new window If resources are limited, FIGO suggests routine screening for preterm preeclampsia by maternal factors and MAP should be performed in all pregnancies, with measurement of PLGF and UTPI reserved for a subgroup who are selected on the basis of their increased risk.[131] A lack of screening availability may lead to a more acute presentation of preeclampsia, with increased risk of clinical sequelae. All women with suspected preeclampsia, including those with nonspecific symptoms such as nausea, vomiting or malaise, should be evaluated for possible HELLP syndrome.
See Gestational hypertension, Preeclampsia and HELLP syndrome.
The US Preventive Services Task Force (USPSTF) recommends screening for gestational diabetes mellitus in asymptomatic pregnant women after 24 weeks' gestation by using a two-step approach (i.e., 50 g oral glucose challenge test [OGCT] followed by a 75 g or 100 g oral glucose tolerance test [OGTT] in those whose OGCT is positive), a one-step approach (i.e., a 75 g OGTT) or fasting plasma glucose measurement.[75] While the USPSTF concludes there are insufficient data to support routine screening prior to 24 weeks, screening may be considered among women who are considered to be high risk for gestational diabetes mellitus. The USPSTF also recommends that for women entering prenatal care after 28 weeks' gestation screening should be performed as soon as possible.
The ACOG supports universal screening of all pregnant women by medical history, clinical risk factors, or laboratory screening test, with screening usually performed at 24 to 28 weeks.[76][77] Two-step laboratory screening is supported by the ACOG. Among women considered to be at high risk for diabetes, early screening may be performed.[75][76][77] If the result of early screening is negative, repeat screening is recommended at 24 to 28 weeks.[76][132] However, if the result of the 50 g OGCT is positive but the follow-up OGTT is negative, the follow-up OGTT is repeated at 24 to 28 weeks without repeating the initial 50 g OGTT.[76] The ACOG does not recommend a one-step OGTT for all pregnant women because of concern that this practice would increase the incidence of a diagnosis of gestational diabetes and resultant healthcare costs without evidence to support an improvement in maternal or newborn outcomes.
In the UK, the National Institute for Health and Care Excellence (NICE) recommends risk assessment for gestational diabetes likelihood using risk factors in a healthy population.[133] The following risk factors are recommended:
BMI (kg/m²) >30
Previous macrosomic baby weighing ≥4.5 kg
Previous pregnancy involving gestational diabetes
Family history of diabetes in a first-degree relative
Ethnicity with a high prevalence of diabetes.
The NICE recommends that all women with at least one risk factor be tested for gestational diabetes using a 2-hour, 75 g oral glucose tolerance test (OGTT). Women with gestational diabetes in a previous pregnancy should be considered for early screening in the first or second trimester, as soon as possible after the first prenatal visit, or alternatively they may perform early self-monitoring of blood glucose. If the early screening test is negative, further screening with an OGTT between 24 and 28 weeks is recommended. For women with all other risk factors, a single OGTT at 24 to 28 weeks is recommended. Additionally, glycosuria of 2+ any time in pregnancy or 1+ on two or more visits should prompt further testing to exclude gestational diabetes.[133]
The ACOG recommends that all pregnant women should be screened at 36+0 to 37+6 weeks' gestation for vaginal-rectal GBS colonization, unless intrapartum prophylaxis is indicated because GBS was previously isolated from the urine in any concentration during pregnancy or the woman had a previous newborn with GBS infection.[78] Women with a positive prenatal GBS result when screened at 36+0 to 37+6 weeks' gestation should receive intrapartum antibiotic prophylaxis, unless they undergo a cesarean birth before the onset of labor and with intact membranes.[78] Women presenting with unknown GBS colonization status when labor starts who are at increased risk of GBS early-onset disease should be offered intrapartum antibiotic prophylaxis.[78] At-risk women include those with: anticipated premature delivery <37 weeks, preterm prelabor rupture of membranes or rupture of membranes 18 hours or longer at term, known positive GBS colonization in a previous pregnancy, or maternal temperature of 100.4°F (38.0°C) or higher.[78] If intra-amniotic infection is suspected, broad-spectrum antibiotic therapy should replace the antibiotic for GBS prophylaxis.[78]
Rhesus (Rh)-negative women should receive Rho (D) immune globulin within 72 hours of exposure to Rh D-positive fetal blood or fetal blood of unknown Rh status if they have any of the following potentially sensitizing events in the first or second trimester:[16]
An ectopic pregnancy
Evacuation of molar pregnancy
Threatened miscarriage or miscarriage
Abortion (medical or surgical)
Any invasive procedures (amniocentesis, chorionic villus sampling)
Antepartum fetal-maternal hemorrhage (abdominal trauma, placental abruption)
Intrauterine fetal death
External cephalic version
If they deliver a baby who is Rh-positive.
Rh-negative women should routinely be given a single prenatal dose of Rho (D) immune globulin at 28 weeks' gestation, followed by a second dose after delivery if the baby is Rh-positive.[13][16] The woman may decline administration if paternity is certain and the father of the pregnancy is known to be Rh-negative.[13][16]
See Rh incompatibility.
Anemia in pregnancy has been defined as hemoglobin (Hb) levels <110 g/L (<11 g/dL) and hematocrit (Hct) levels <33% in the first and third trimester, and Hb levels <105 g/L (10.5 g/dL) and Hct levels <32% in the second-trimester.[134][135]
Anemia in pregnancy can be dilutional (physiological), due to iron, folate or other vitamin deficiency, a result of thalassemias, or result from other causes. Iron deficiency is the most common cause of anemia in pregnancy, thus the risk of iron deficiency should be considered in all pregnant women. The CDC recommends daily elemental iron supplementation for prophylaxis or treatment of iron-deficiency anemia.[135] Absorption of iron can be facilitated with vitamin C.[134][135][136]
Obese women should also be informed that they are at risk for pregnancy complications, including gestational hypertension, gestational diabetes mellitus, cardiac disease, pulmonary disease, obstructive sleep apnea, and cesarean delivery.[42][43] Regular aerobic exercise during pregnancy may reduce some of these risks. Although evidence indicates that monitored physical activity seems to limit gestational weight gain among overweight or obese pregnant women, effects on maternal and neonatal health are unproven.[137] Prenatal dietary modifications in obese pregnant women can reduce maternal weight gain during pregnancy without adversely influencing neonatal birth weight.[138]
Obese patients may also be at increased risk for venous thromboembolism, particularly if other risk factors are present.[42][139] Thromboprophylaxis should be considered on an individual basis based upon a patient’s risk profile. Prenatal consultation with an anesthesiologist should be considered for patients with Class III obesity (BMI 40 or above), to discuss interventions (such as an early epidural) that may limit intrapartum risk.[42]
Obesity further appears to be associated with an increased risk of selected fetal malformations and stillbirth, as well as a more limited ability to sonographically detect anomalies during the prenatal period.[140]
See Obesity in adults.
Post-term pregnancy is defined as a gestation that has extended to or beyond 42+0 weeks' gestation from the last menstrual period.[141][142] Accurate dating of pregnancy is important for diagnosis and management of late- and post-term pregnancies.[93] The risks of a prolonged pregnancy should be discussed with the pregnant woman so they can make a decision between induction or waiting for spontaneous onset of labor. There is a significantly reduced likelihood of perinatal mortality at 41 weeks' gestation, compared with 42 weeks' gestation, with risks increasing as the length of the post-term pregnancy increases.[143] Although differences exist between published practice guidelines, women may be offered induction of labor by 41 weeks' gestation (late-term gestation) to avoid risks of post-term pregnancy.[142][144][145][146] In suboptimally dated pregnancies, if the gestational age is uncertain, the best clinical estimate should be used to determine a gestational age of 41 weeks’ gestation; antepartum fetal surveillance can be considered at 39 to 40 weeks’ gestation.[97] If pregnancy continues beyond 41 weeks, fetal testing should include at least a non-stress test and an assessment of amniotic fluid volume twice weekly.[144]
Use of this content is subject to our disclaimer