Occurrence of a sudden, severe headache is characteristic of SAH.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
It is the most important clue to diagnosis and is often described as "the worst ever headache." Prompt diagnostic workup and evaluation are recommended to diagnose/exclude aneurysmal SAH (aSAH) and to minimize morbidity and mortality.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
History and exam
The first priority should be an urgent assessment of level of consciousness and need for cardiopulmonary resuscitation and/or ventilatory support.[38]Axelrod KA, Diringer MN. Medical management of subarachnoid hemorrhage. In: Bhardwaj A, Alkayed NJ, Kirsch JR, et al, eds. Acute stroke: bench to bedside. New York, NY: Informa Healthcare; 2006. History-taking (from the patient and/or relatives) may reveal risk factors of smoking, cocaine use, hypertension, family history of SAH, connective tissue disorders, or autosomal dominant polycystic kidney diseases. Consciousness level should be assessed using the Glasgow Coma Scale (GCS).[39]Steiner T, Juvela S, Unterberg A, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013 Feb 7;35(2):93-112.
https://www.karger.com/Article/FullText/346087
http://www.ncbi.nlm.nih.gov/pubmed/23406828?tool=bestpractice.com
On admission, up to two-thirds of people with SAH have a depressed level of consciousness, half of whom are in a coma.[40]Brilstra EH, Rinkel GJ, Algra A, et al. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology. 2000 Dec 12;55(11):1656-60.
http://www.ncbi.nlm.nih.gov/pubmed/11113219?tool=bestpractice.com
Physical exam can be normal, or there can be altered level of consciousness, agitation, altered mental state, meningismus, and focal findings. A poor level of awareness and seizures on presentation are risk factors for aspiration. A large hemorrhage burden and the presence of a subdural hematoma are associated with the occurrence of seizures after aneurysm rupture.[41]Ibrahim GM, Fallah A, Macdonald RL. Clinical, laboratory, and radiographic predictors of the occurrence of seizures following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2013 Aug;119(2):347-52.
http://www.ncbi.nlm.nih.gov/pubmed/23581590?tool=bestpractice.com
Photophobia, nausea, and vomiting are common symptoms. A full neurologic exam should be performed with special attention to pupillary reaction. Fixed and dilated pupils in comatose patients are associated with a poor prognosis, especially when present bilaterally.[42]Clusmann H, Schaller C, Schramm J. Fixed and dilated pupils after trauma, stroke, and previous intracranial surgery: management and outcome. J Neurol Neurosurg Psychiatry. 2001 Aug;71(2):175-81.
https://pmc.ncbi.nlm.nih.gov/articles/PMC1737504
http://www.ncbi.nlm.nih.gov/pubmed/11459888?tool=bestpractice.com
Intraocular hemorrhages (secondary to increased intracranial pressure) are seen in 10% to 40% of patients with SAH.[43]McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson's syndrome: frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004 Mar;75(3):491-3.
https://jnnp.bmj.com/content/75/3/491.long
http://www.ncbi.nlm.nih.gov/pubmed/14966173?tool=bestpractice.com
They cause visual loss in the affected eye. This is associated with worse prognosis and increased mortality.[43]McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson's syndrome: frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004 Mar;75(3):491-3.
https://jnnp.bmj.com/content/75/3/491.long
http://www.ncbi.nlm.nih.gov/pubmed/14966173?tool=bestpractice.com
Cranial nerve palsies may be present. Isolated dilation of one pupil and loss of the pupillary light reflex may indicate brain herniation as a result of rising intracranial pressure, caused by an intraparenchymal component to the hemorrhage or hydrocephalus. A poor neurologic status on admission seems to predict cardiac abnormalities thought to be secondary to overwhelming sympathetic activation.[44]Tung P, Kopelnik A, Banki N, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004 Feb;35(2):548-51.
http://www.ncbi.nlm.nih.gov/pubmed/14739408?tool=bestpractice.com
[45]Zaroff JG, Rordorf GA, Newell JB, et al. Cardiac outcome in patients with subarachnoid hemorrhage and electrocardiographic abnormalities. Neurosurgery. 1999 Jan;44(1):34-9.
http://www.ncbi.nlm.nih.gov/pubmed/9894961?tool=bestpractice.com
[46]Zaroff JG, Rordorf GA, Ogilvy CS, et al. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr. 2000 Aug;13(8):774-9.
http://www.ncbi.nlm.nih.gov/pubmed/10936822?tool=bestpractice.com
[47]Jain R, Deveikis J, Thompson BG. Management of patients with stunned myocardium associated with subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2004 Jan;25(1):126-9.
http://www.ncbi.nlm.nih.gov/pubmed/14729541?tool=bestpractice.com
Close monitoring of vital signs should be instituted, including blood pressure, heart rate and rhythm, and respiratory rate.[48]Al-Shahi R, White PM, Davenport RJ, et al. Clinical review: subarachnoid haemorrhage. BMJ. 2006 Jul 29;333(7561):235-40.
Serum tests and ECG
Complete blood count, serum electrolytes, and clotting profile should be ordered in the initial workup in addition to serum troponin I. Half of patients have an abnormal ECG on admission.[49]Solenski NJ, Haley EC Jr, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995 Jun;23(6):1007-17.
http://www.ncbi.nlm.nih.gov/pubmed/7774210?tool=bestpractice.com
Abnormalities include arrhythmias, prolonged QTc, and ST segment/T wave abnormalities.[44]Tung P, Kopelnik A, Banki N, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004 Feb;35(2):548-51.
http://www.ncbi.nlm.nih.gov/pubmed/14739408?tool=bestpractice.com
[49]Solenski NJ, Haley EC Jr, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995 Jun;23(6):1007-17.
http://www.ncbi.nlm.nih.gov/pubmed/7774210?tool=bestpractice.com
[50]Deibert E, Barzilai B, Braverman AC, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg. 2003 Apr;98(4):741-6.
http://www.ncbi.nlm.nih.gov/pubmed/12691398?tool=bestpractice.com
[51]Bulsara KR, McGirt MJ, Liao L, et al. Use of the peak troponin value to differentiate myocardial infarction from reversible neurogenic left ventricular dysfunction associated with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003 Mar;98(3):524-8.
http://www.ncbi.nlm.nih.gov/pubmed/12650423?tool=bestpractice.com
Computed tomography (CT) and lumbar puncture (LP)
Suspicion of SAH based on a history of sudden, severe headaches is sufficient to order an emergency noncontrast brain CT as the first test.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
[52]American College of Radiology. ACR Appropriateness Criteria®: headache. 2022 [internet publication].
https://acsearch.acr.org/docs/69482/Narrative
However, the specific workup required depends on the time of presentation from symptom onset and the patient’s neurologic status.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
Some, but not all patients will require additional investigation:[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
In patients who present <6 hours from symptom onset of acute onset severe headache, a noncontrast head CT performed on a high-quality scanner and interpreted by a board-certified neuroradiologist is reasonable to diagnose/exclude aneurysmal SAH (aSAH).
In patients with acute onset of severe headache who present >6 hours from symptom onset or who have a new neurologic deficit, a negative noncontrast head CT should prompt a lumbar puncture (LP) to diagnose/exclude aSAH.
In patients with acute onset of severe headache without a new neurologic deficit, application of the Ottawa SAH Rule may be reasonable to identify those at high risk for aSAH.
In atypical presentations, such as primary neck pain, syncope, seizure, or new focal neurologic deficit where there is a high suspicion of SAH, appropriate imaging and workup should still be considered.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
Ottawa SAH rule[53]Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA. 2013 Sep 25;310(12):1248-55.
https://www.doi.org/10.1001/jama.2013.278018
http://www.ncbi.nlm.nih.gov/pubmed/24065011?tool=bestpractice.com
For alert patients >15 years of age with new severe nontraumatic headache reaching maximum intensity within 1 hour. Patients require additional investigation for SAH if they meet any of the following criteria:
Ages ≥40 years
Neck pain or stiffness
Witnessed loss of consciousness
Onset during exertion
Thunderclap headache (instantly peaking pain)
Limited neck flexion on exam
CT: Thin cuts should be ordered (3-5 mm); otherwise, small, thin collections of blood might be missed. Subarachnoid blood will appear hyperdense (white) in the basal cisterns, major fissures, and sulci.[54]Latchaw RE, Silva P, Falcone SF. The role of CT following aneurysmal rupture. Neuroimaging Clin N Am. 1997 Nov;7(4):693-708.
http://www.ncbi.nlm.nih.gov/pubmed/9336494?tool=bestpractice.com
Detection of SAH on CT depends on density of blood, quantity of SAH, and timing of CT from ictus. A small quantity of blood in the subarachnoid space may be missed, and blood with hemoglobin below 10 g/dL may not be visible.[54]Latchaw RE, Silva P, Falcone SF. The role of CT following aneurysmal rupture. Neuroimaging Clin N Am. 1997 Nov;7(4):693-708.
http://www.ncbi.nlm.nih.gov/pubmed/9336494?tool=bestpractice.com
Nonetheless, the advent of third-generation CT scanners has dramatically improved the sensitivity of detecting subarachnoid blood, reaching 100% when performed within 6 hours of headache onset and read by experienced neuroradiologists.[55]Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011 Jul 18;343:d4277.
https://www.bmj.com/content/343/bmj.d4277.long
http://www.ncbi.nlm.nih.gov/pubmed/21768192?tool=bestpractice.com
[56]Backes D, Rinkel GJ, Kemperman H, et al. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke. 2012 Aug;43(8):2115-9.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.112.658880
http://www.ncbi.nlm.nih.gov/pubmed/22821609?tool=bestpractice.com
[57]Dubosh NM, Bellolio MF, Rabinstein AA, et al. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke. 2016 Mar;47(3):750-5.
http://www.ncbi.nlm.nih.gov/pubmed/26797666?tool=bestpractice.com
The aneurysm rupture site can be predicted, though inconsistently, from patterns of blood accumulation on CT (thick collection in fissures) or parenchymal hematoma.[58]van der Jagt M, Hasan D, Bijvoet HW, et al. Validity of prediction of the site of ruptured intracranial aneurysms with CT. Neurology. 1999 Jan 1;52(1):34-9.
http://www.ncbi.nlm.nih.gov/pubmed/9921845?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: CT brain showing subarachnoid hemorrhage from a ruptured posterior cerebral artery aneurysm (1 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].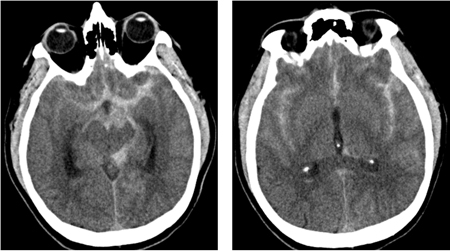 [Figure caption and citation for the preceding image starts]: CT brain showing subarachnoid hemorrhage from a ruptured posterior cerebral artery aneurysm (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: CT brain showing subarachnoid hemorrhage from a ruptured posterior cerebral artery aneurysm (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
Lumbar puncture: Four tubes of cerebrospinal fluid (CSF) should be collected and examined for gross blood. A serial count of red blood cells (RBCs) in tubes 1 to 4 is not sufficiently accurate to distinguish SAH from a traumatic LP. Visual inspection for xanthochromia is unreliable. Spectrophotometric analysis of hemoglobin degradation products is most reliable.[59]Cruickshank A, Auld P, Beetham R, et al; UK NEQAS Specialist Advisory Group for EQA of CSF Proteins and Biochemistry. Revised national guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann Clin Biochem. 2008 May;45(Pt 3):238-44.
http://journals.sagepub.com/doi/full/10.1258/acb.2008.007257
http://www.ncbi.nlm.nih.gov/pubmed/18482910?tool=bestpractice.com
RBCs in the subarachnoid space start lysing approximately 12 hours after the bleed. Lysed RBCs will impart a xanthochromic (faint, yellow tinge) appearance to the CSF. However, high protein content in CSF or contamination with iodine used for disinfection can cause CSF to look xanthochromic.[60]Wijdicks EF, Kallmes DF, Manno EM, et al. Subarachnoid hemorrhage: neurointensive care and aneurysm repair. Mayo Clin Proc. 2005 Apr;80(4):550-9.
http://www.ncbi.nlm.nih.gov/pubmed/15819296?tool=bestpractice.com
Further imaging
After SAH is confirmed by CT or LP, further imaging tests should be ordered.[67]American College of Radiology. ACR Appropriateness Criteria®: cerebrovascular diseases - aneurysm, vascular malformation, and subarachnoid hemorrhage. 2021 [internet publication].
https://acsearch.acr.org/docs/3149013/Narrative
http://www.ncbi.nlm.nih.gov/pubmed/34794589?tool=bestpractice.com
Digital subtraction angiography (DSA) is the most accurate imaging technique used to diagnose aneurysms. CT angiography (CTA) and magnetic resonance angiography (MRA) are noninvasive imaging methods that have been compared with DSA.[67]American College of Radiology. ACR Appropriateness Criteria®: cerebrovascular diseases - aneurysm, vascular malformation, and subarachnoid hemorrhage. 2021 [internet publication].
https://acsearch.acr.org/docs/3149013/Narrative
http://www.ncbi.nlm.nih.gov/pubmed/34794589?tool=bestpractice.com
[68]Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003 Mar;52(3):624-31.
http://www.ncbi.nlm.nih.gov/pubmed/12590688?tool=bestpractice.com
[69]Hoh BL, Cheung AC, Rabinov JD, et al. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004 Jun;54(6):1329-40.
http://www.ncbi.nlm.nih.gov/pubmed/15157289?tool=bestpractice.com
[70]Jayaraman MV, Mayo-Smith WW, Tung GA, et al. Detection of intracranial aneurysms: multi-detector row CT angiography compared with DSA. Radiology. 2004 Feb;230(2):510-8.
http://www.ncbi.nlm.nih.gov/pubmed/14699177?tool=bestpractice.com
[71]Kouskouras C, Charitanti A, Giavroglou C, et al. Intracranial aneurysms: evaluation using CTA and MRA. Correlation with DSA and intraoperative findings. Neuroradiology. 2004 Oct;46(10):842-50.
http://www.ncbi.nlm.nih.gov/pubmed/15448952?tool=bestpractice.com
CTA is widely available and often is the next diagnostic test performed when SAH is diagnosed with noncontrast CT.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
A meta-analysis reported CTA to have a sensitivity of 92.7% and specificity of 77.2%, though another reported sensitivity and specificity surpassing 95%, especially when newer-generation multidetector scanners were used.[68]Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003 Mar;52(3):624-31.
http://www.ncbi.nlm.nih.gov/pubmed/12590688?tool=bestpractice.com
[69]Hoh BL, Cheung AC, Rabinov JD, et al. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004 Jun;54(6):1329-40.
http://www.ncbi.nlm.nih.gov/pubmed/15157289?tool=bestpractice.com
[72]van Gelder JM. Computed tomographic angiography for detecting cerebral aneurysms: implications of aneurysm size distribution for the sensitivity, specificity, and likelihood ratios. Neurosurgery. 2003 Sep;53(3):597-605.
http://www.ncbi.nlm.nih.gov/pubmed/12943576?tool=bestpractice.com
[73]Menke J, Larsen J, Kallenberg K. Diagnosing cerebral aneurysms by computed tomographic angiography: meta-analysis. Ann Neurol. 2011 Apr;69(4):646-54.
http://www.ncbi.nlm.nih.gov/pubmed/21391230?tool=bestpractice.com
CTA head sensitivity for detecting aneurysm decreases for aneurysms <3 mm in size in the setting of diffuse SAH, and for aneurysms occurring adjacent to an osseous structure.[67]American College of Radiology. ACR Appropriateness Criteria®: cerebrovascular diseases - aneurysm, vascular malformation, and subarachnoid hemorrhage. 2021 [internet publication].
https://acsearch.acr.org/docs/3149013/Narrative
http://www.ncbi.nlm.nih.gov/pubmed/34794589?tool=bestpractice.com
CTA may be sufficient to rule out a vascular cause of SAH when the location of hemorrhage is isolated to the perimesencephalic region with follow-up catheter-directed angiography indicated in CTA negative diffuse or peripheral SAHs.[67]American College of Radiology. ACR Appropriateness Criteria®: cerebrovascular diseases - aneurysm, vascular malformation, and subarachnoid hemorrhage. 2021 [internet publication].
https://acsearch.acr.org/docs/3149013/Narrative
http://www.ncbi.nlm.nih.gov/pubmed/34794589?tool=bestpractice.com
[74]Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: When is digital subtraction angiography still needed? AJNR Am J Neuroradiol. 2010 Apr;31(4):696-705.
http://www.ajnr.org/content/31/4/696.long
http://www.ncbi.nlm.nih.gov/pubmed/19942709?tool=bestpractice.com
However, there remains equipoise concerning the appropriate diagnostic pathway for a perimesencephalic distribution of SAH with CTA alone versus catheter-based DSA.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
Similarly, meta-analysis has shown that MRA has a sensitivity of 95% and specificity of 89%.[75]Sailer AM, Wagemans BA, Nelemans PJ, et al. Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke. 2014 Jan;45(1):119-26.
http://www.ncbi.nlm.nih.gov/pubmed/24326447?tool=bestpractice.com
Limitations of MRA head include required safety screening and relatively long acquisition time in urgent clinical scenarios.[67]American College of Radiology. ACR Appropriateness Criteria®: cerebrovascular diseases - aneurysm, vascular malformation, and subarachnoid hemorrhage. 2021 [internet publication].
https://acsearch.acr.org/docs/3149013/Narrative
http://www.ncbi.nlm.nih.gov/pubmed/34794589?tool=bestpractice.com
DSA is considered the gold-standard modality for the evaluation of cerebrovascular anatomy and aneurysm geometry and can aid in decision-making on the choice of optimal treatment modality.[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
AHA/ASA recommends that in patients with spontaneous SAH with high level of concern for aneurysmal source and a negative or inconclusive CT angiography (CTA), digital subtraction angiography (DSA) should be performed to diagnose/exclude cerebral aneurysm(s).[37]Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023 Jul;54(7):e314-70.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000436?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/37212182?tool=bestpractice.com
For diffuse SAH, DSA is indicated for evaluation regardless of CTA results because small aneurysms or other vascular lesions may not be fully appreciated or defined on CTA imaging owing to limitations in spatial resolution.[74]Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: When is digital subtraction angiography still needed? AJNR Am J Neuroradiol. 2010 Apr;31(4):696-705.
http://www.ajnr.org/content/31/4/696.long
http://www.ncbi.nlm.nih.gov/pubmed/19942709?tool=bestpractice.com
[76]Catapano JS, Lang MJ, Koester SW, et al. Digital subtraction cerebral angiography after negative computed tomography angiography findings in non-traumatic subarachnoid hemorrhage. J Neurointerv Surg. 2020 May;12(5):526-30.
https://jnis.bmj.com/content/12/5/526.long
http://www.ncbi.nlm.nih.gov/pubmed/31685693?tool=bestpractice.com
[77]Heit JJ, Pastena GT, Nogueira RG, et al. Cerebral angiography for evaluation of patients with CT angiogram-negative subarachnoid hemorrhage: an 11-year experience. AJNR Am J Neuroradiol. 2016 Feb;37(2):297-304.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7959954
http://www.ncbi.nlm.nih.gov/pubmed/26338924?tool=bestpractice.com
[78]Howard BM, Hu R, Barrow JW, et al. Comprehensive review of imaging of intracranial aneurysms and angiographically negative subarachnoid hemorrhage. Neurosurg Focus. 2019 Dec;47(6):E20.
https://thejns.org/focus/view/journals/neurosurg-focus/47/6/article-pE20.xml
http://www.ncbi.nlm.nih.gov/pubmed/31786554?tool=bestpractice.com
[79]Rustemi O, Alaraj A, Shakur SF, et al. Detection of unruptured intracranial aneurysms on noninvasive imaging. Is there still a role for digital subtraction angiography? Surg Neurol Int. 2015;6:175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4665160
http://www.ncbi.nlm.nih.gov/pubmed/26674519?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: CT brain showing subarachnoid hemorrhage from a ruptured posterior cerebral artery aneurysm (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: CT brain showing subarachnoid hemorrhage from a ruptured posterior cerebral artery aneurysm (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
