Complications
Rebleeding is an important complication. The incidence is 5.7% during the first 72 hours, and cumulative risk approaches 22% at 1 month after SAH.[11][12] Patients with modified Fisher grade bleeding of III and IV are more likely to rebleed.[94][95][175] Rebleeding is responsible for 8% to 22% of mortality and is significantly associated with poor outcome.[11][12][49]
Routine use of antifibrinolytic therapy does not improve functional outcome.[37][113] Antifibrinolytic therapy is associated with increased risk of cerebral ischemia.[176][177] Conversely, ultra-early administration of antifibrinolytic therapy might reduce the risk of rebleeding, but this effect has not been consistent across trials.[37][177][178][179] Prompt obliteration of the ruptured aneurysm is the only treatment proven to be effective to reduce the likelihood of rebleeding and thus early mortality.[37]
Acute hydrocephalus (HCP) occurs in 15% to 20% of patients during the first 72 hours and is an obstructive HCP.[11][12][180] Its occurrence is related to presence of intraventricular blood and, to a lesser extent, thick cisternal blood collection.[180][181] The mortality rate in SAH patients with HCP is higher than in those without it.[180]
In patients with aSAH and acute symptomatic hydrocephalus, urgent cerebrospinal fluid (CSF) diversion (external ventricular drain [EVD] and/or lumbar drainage) should be performed.[37] The use of prophylactic antibiotics with EVD is not established but is commonly adopted.[60] The optimum modes of CSF drainage (intermittent vs. continuous), EVD weaning (rapid vs. gradual), and EVD wean timing (following aneurysm securement vs. time of vasospasm risk) are uncertain.[7][113][182] It is unclear whether microsurgical fenestration of the lamina terminalis during surgical clipping of the aneurysm protects against the need for a permanent shunt in patients with hydrocephalus.[183][Figure caption and citation for the preceding image starts]: Communicating hydrocephalus in the setting of subarachnoid hemorrhage; note dilation of fourth and temporal horns of lateral ventriclesCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].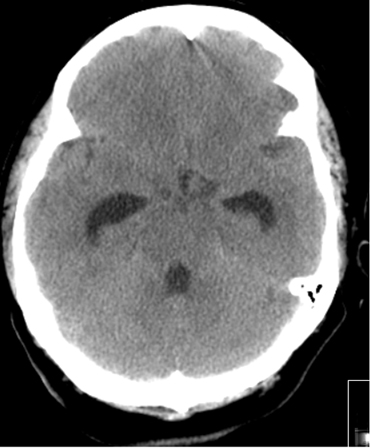
Vasospasm is a delayed, focal, or diffuse narrowing of large capacitance vessels of the circle of Willis. It accounts for 23% of deaths related to SAH.[49] The pathophysiology of vasospasm is poorly understood, a fact compounded by the use of varying nomenclature and definitions; however, it is believed to result from a delayed and reversible vasculopathy, impaired autoregulatory function, and hypovolemia, culminating in a global or regional reduction of cerebral perfusion, which when below a certain threshold causes ischemia.[185] In addition, low hemoglobin may impair oxygen delivery to brain regions with precarious perfusion, further compounding this problem.[186][187][188][189][190] Presence of oxyhemoglobin in the subarachnoid space seems to be necessary, and an inflammatory response is implicated in the pathogenesis.[191][192][193]
Vasospasm develops between days 4 and 14 after SAH and is seen on angiography in 50% to 70% of cases. Half of these patients develop delayed cerebral ischemia (DCI) secondary to reduced regional or overall cerebral blood flow (CBF).[194] If untreated, DCI progresses to permanent cerebral infarction in 50% of cases. Ischemic deficits may also be seen in the absence of discrete angiographic vasospasm. This is believed to be due, in part, to altered autoregulation of distal cerebral vessels, microthrombi in such vessels, and/or cortical spreading depolarization.[195] Risk factors for DCI are a poor clinical condition on admission, quantity and duration of exposure to subarachnoid blood, thick blood collections in cisterns and fissures, intraventricular blood, and duration of unconsciousness.[176][181][196][197][198] Although the presence of blood in the subarachnoid space is necessary to the development of vasospasm, surgical clipping, during which most of the subarachnoid blood is washed out, does not seem to carry a lesser risk of vasospasm than endovascular coiling.[199][200][Figure caption and citation for the preceding image starts]: Severe vasospasm of distal left internal carotid artery and proximal middle and anterior cerebral arteries before (A) and after (B) intra-arterial infusion of nicardipine and transluminal balloon angioplastyCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].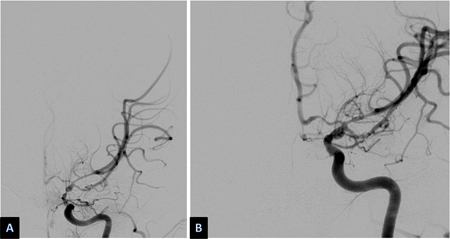 [Figure caption and citation for the preceding image starts]: Left frontal infarct (arrows) in a patient with subarachnoid hemorrhage-related vasospasmCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Left frontal infarct (arrows) in a patient with subarachnoid hemorrhage-related vasospasmCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].
The diagnosis of DCI is a clinical one made after excluding rebleeding, hydrocephalus, seizures, electrolyte imbalances, and other metabolic disturbances. Clinically, patients develop alteration of consciousness or acute to subacute fluctuating focal neurologic deficits.[11][12][192]
However, the diagnosis can be challenging, and although serial neurologic exams are important, they are of limited value in patients with high-grade aSAH.[37] In patients with suspected vasospasm or limited neurologic exam, CTA or CT perfusion (CTP) can be useful to detect vasospasm and predict DCI.[37] CTP is noninvasive and, most importantly, measure perfusion, not merely arterial diameter or flow velocities.[201] CT angiography (CTA) and CTP have shown excellent accuracy in diagnosing vasospasm. CTA vasospasm scores are direct predictors of DCI and poor neurologic outcome, and CTP allows early prediction of perfusion abnormalities.[37] However, these CT imaging techniques do not allow for therapeutic intervention (e.g., transluminal balloon angioplasty and intra-arterial vasodilators) in the same way that angiography permits.[202] CTA is highly correlated to conventional angiography for larger proximal intracranial vessels with decreasing correlation in the smaller more distal arteries.[67][203] CTA shows high sensitivity (91%) for detecting central vasospasm when symptoms develop.[37][204] Transcranial Doppler (TCD) ultrasound monitoring is a noninvasive, safe bedside neuromonitoring technique that allows repetitive and dynamic assessment of vasospasm after SAH.[37] It is reasonable to use TCD to detect vasospasm and predict DCI but it is less reliable than angiography.[37][205][206] TCD is operator dependent, can be limited by patient anatomy (poor temporal bone window), and can be affected by other physiologic measures (such as heart rate and BP).[37][207][208] In patients with high-grade aSAH, continuous EEG (cEEG) monitoring can be useful to predict DCI.[37][209]
In patients without symptomatic vasospasm, induction of hypertension and hypervolemia is potentially harmful because of the association with excess morbidity including cerebral edema, hemorrhagic transformation in areas of infarction, reversible leukoencephalopathy, myocardial infarction, and congestive heart failure.[37][39][106][107][108][109] Instead, maintaining euvolemia can be beneficial in preventing DCI and improving functional outcomes.[37] However, in patients with SAH and symptomatic vasospasm, elevating systolic BP values may be reasonable to reduce the progression and severity of DCI.[37][210][211] Symptomatic patients should be kept hypervolemic (central venous pressure ≥8 cm H₂O) and hypertension induced using vasopressors. Isotonic fluids are used. Hypertension should be titrated to a mean arterial pressure (MAP) of at least 15% higher than the patient's average MAP, until clinical improvement or adverse effects occur. Clinicians should be aware of the possibility of posterior reversible encephalopathy syndrome development in the context of increased blood pressure. The diagnosis is usually heralded by clinical deterioration and confirmed by magnetic resonance imaging.[212]
Triple H (hypertension, hypervolemia, and hemodilution), also known as hyperdynamic or hypervolemic hypertensive therapy (HHT), is safe, even in patients with prior cardiac disease.[194] Clinical improvement can be dramatic, but large prospective outcome studies of HHT are lacking.[108] A systematic review of uncontrolled studies has suggested that hypertension seems to be more effective in increasing CBF than hemodilution or hypervolemia.[213] Randomized controlled studies are difficult to conduct, given the multiplicity of factors that affect outcome in SAH.
Endovascular techniques such as transluminal balloon angioplasty and intra-arterial vasodilators will reverse arterial narrowing, though clinical improvement is not consistent.[214][215] ASA/AHA recommends that in patients with aSAH and severe vasospasm, cerebral angioplasty may be reasonable to reverse cerebral vasospasm and reduce the progression and severity of DCI.[37] There is some evidence to support early angioplasty (within 2 hours of symptom onset) in providing sustained clinical improvement.[216] Increased age and poor neurologic status at presentation are predictive of poor clinical outcome after angioplasty.[217] Enteral nimodipine, a calcium-channel antagonist, is given for vasospasm prophylaxis.[37] It reduces risk of poor outcome and secondary ischemia after aneurysmal SAH.[218][219]
[  ]
Despite their wide use, studies of corticosteroids in SAH have failed to show positive effects on DCI or overall outcome.[220] Tirilazad, a nonglucocorticoid 21 amino-steroid free-radical scavenger, was studied in several controlled trials for prevention of vasospasm. It was well tolerated but had inconsistent effect on overall outcome across the different studies.[221][222][223][224][225]
[
]
Despite their wide use, studies of corticosteroids in SAH have failed to show positive effects on DCI or overall outcome.[220] Tirilazad, a nonglucocorticoid 21 amino-steroid free-radical scavenger, was studied in several controlled trials for prevention of vasospasm. It was well tolerated but had inconsistent effect on overall outcome across the different studies.[221][222][223][224][225]
[  ]
Studies have investigated the use of simvastatin and pravastatin in SAH with mixed results.[226][227][228][229] Routine use of statin therapy to improve outcomes is not recommended.[37] Studies investigating the use of antiplatelet agents in SAH, especially following endovascular coiling, have yielded conflicting results on the risk of DCI and overall outcome. Those conflicting findings, together with an increased risk of hemorrhagic complications, have resulted in less than universal adoption of this practice.[230][231]
[
]
Studies have investigated the use of simvastatin and pravastatin in SAH with mixed results.[226][227][228][229] Routine use of statin therapy to improve outcomes is not recommended.[37] Studies investigating the use of antiplatelet agents in SAH, especially following endovascular coiling, have yielded conflicting results on the risk of DCI and overall outcome. Those conflicting findings, together with an increased risk of hemorrhagic complications, have resulted in less than universal adoption of this practice.[230][231]
[  ]
[Figure caption and citation for the preceding image starts]: Distal left vertebral and basilar arteries spasm before (left) and after (right) intra-arterial Infusion of nicardipineCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].
]
[Figure caption and citation for the preceding image starts]: Distal left vertebral and basilar arteries spasm before (left) and after (right) intra-arterial Infusion of nicardipineCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].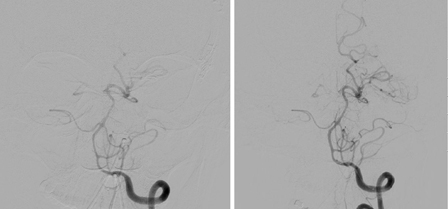
The effect on vasospasm and DCI of pharmacologic compounds with controlled-release forms has drawn much interest. Nicardipine prolonged-release implants are placed in the subarachnoid space at the time of surgical clipping of aneurysm. Small case series and a randomized double-blind trial using such implants have reported a lower than expected incidence of DCI.[232][233][234][235]
Other drugs used in an intracranial, controlled-release system include papaverine, fasudil, and nitric oxide donors.[236] Larger controlled trials are needed before widespread use of this technique. Papaverine, although a historically effective vasodilator, is generally avoided because of the risk of neurotoxicity.[37][237] The use of thrombolytic agents instilled during surgery and/or after securing the aneurysm into the intrathecal space could potentially be beneficial. They rid the subarachnoid space of blood necessary for the development of vasospasm. A meta-analysis concluded that while such compounds could improve outcome, the analyzed studies had limitations, including considerable risk of bias.[238] Another intervention that rids the subarachnoid space of blood is lumbar drainage (LD) of cerebral spinal fluid. Trials of LD show a benefit on the incidence of vasospasm and DCI, but no effect on functional outcomes or mortality at 6 months.[239] Studies of other forms of intracranial delivery of nicardipine (intrathecal, intraventricular and intracisternal) have yielded mixed results. In the absence of positive randomized controlled trials, these alternative forms of intracranial delivery are reserved for severe cases of vasospasm and DCI where other therapies have failed.[235]
It is unknown whether angiographic, asymptomatic vasospasm needs to be treated.[240] Except for one retrospective study using albumin, prophylactic volume expansion in asymptomatic patients has failed to show an impact on outcome.[107][108][241]
Arrhythmias and nonspecific ECG changes are common.[44][49][50][51] Ventricular wall motion abnormalities occur in 27% of patients. An apex-sparing pattern is seen in half of affected patients. These abnormalities rarely cause cardiac failure and are almost always reversible.[47][242][243][Figure caption and citation for the preceding image starts]: ECG done on admission of a patient with subarachnoid hemorrhage; note peaked, tall T waves (1 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
Treatment of cardiac failure is supportive. Beta-blockers can be administered for ischemic-type ECG changes accompanied by troponin leaks; however, there is no evidence in support of this practice. [Figure caption and citation for the preceding image starts]: Same patient, 24 hours later; note normalization of T waves (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].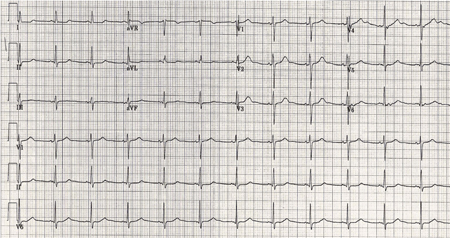
Pulmonary edema occurs in up to 23% of patients.
Pulmonary edema is severe, requiring ventilatory support in 6% of cases.
It is debated whether it is an independent predictor of outcome or is merely associated with severity of SAH.[244][245][246][247]
Hyperglycemia on admission, during aneurysm surgery, or within 72 hours of SAH presentation has been associated with vasospasm, delayed cerebral ischemia, unfavorable short-term and long-term functional outcomes, and risk of death in both patients with diabetes and those without diabetes in multiple studies.[37][247][248][249][250] AHA/ASA recommend that in patients with SAH, effective glycemic control, strict hyperglycemia management, and avoidance of hypoglycemia are reasonable to improve outcome.[37] However, data are conflicting on what glycemic threshold should be targeted, what monitoring and treatment intensities should be used, and whether all these affect outcome.[37] It remains to be determined whether tight glycemic control could lead to systemic or cerebral hypoglycemia and metabolic crisis in the acutely injured brain and potentially worsen brain injury and outcome.[37] Until more robust evidence exists for treating hyperglycemia in SAH, one should refrain from some aggressive management strategies (i.e., continuous insulin infusion) used in critically ill patients.[251]
Recent studies using EEG monitoring suggest a seizure incidence of 7.8% to 15.2%.[37][170][171][172] The cumulative incidence of epilepsy (defined as 2 or more unprovoked seizures) is 12% at 5 years.[173] It is independently associated with poor functional recovery and quality of life.[172][174] The risk of seizures is significantly lower after coil embolization than following surgical clipping of aneurysm.[37]
Risk factors for the development of early seizures associated with SAH include clinical grade (Hunt and Hess [HH] grade ≥3), presence of a middle cerebral artery aneurysm, and hydrocephalus.[37] Continuous EEG (cEEG) monitoring is reasonable to detect seizures in patients with SAH and either fluctuating neurologic exam, depressed mental state, ruptured middle cerebral artery (MCA) aneurysm, high-grade SAH, intracranial hemorrhage (ICH), hydrocephalus, or cortical infarction.[37]
Prophylactic use of anticonvulsants following SAH is controversial.[114][115]
US guidelines suggest that prophylactic anticonvulsants may be considered in patients with SAH and high-seizure-risk features (i.e., ruptured MCA aneurysm, high-grade SAH, ICH, hydrocephalus, and cortical infarction).[37] These guidelines recommend against the routine use of anticonvulsants in patients with SAH without high-seizure-risk features since phenytoin for seizure prevention and/or seizure prophylaxis is associated with excess morbidity and mortality.[37][88][116] Short-course treatment may be adequate prophylaxis, and some evidence suggests that it is better tolerated than a longer course.[87][117] Routine long-term use of anticonvulsants is not recommended, but may be considered for patients with known risk factors for delayed seizure disorder.[37] In patients with SAH who present with seizures, treatment with anticonvulsants for ≤7 days is reasonable to reduce seizure-related complications in the perioperative period.[37] In patients with SAH without prior epilepsy who present with seizures, treatment with anticonvulsants beyond 7 days is not effective for reducing future SAH-associated seizure risk.[37][39][114]
Fever in the neurologic intensive care unit (NICU) appears to be an independent predictor of poor outcome.[37] It is associated with longer ICU stay and overall length of hospital stay.[252] Intraventricular catheterization is a risk factor for unexplained fever in the NICU. SAH increases the risk of developing infectious and unexplained fever, which could be associated with a worse outcome.[253][254] An infectious etiology should always be sought. Blood, sputum, urine, and cerebrospinal fluid (if applicable) samples should be obtained for Gram staining and culture.
If fever occurs, antibiotics should be withheld until there is clear evidence of an infection. In patients with SAH with fever refractory to antipyretic medications, the effectiveness of therapeutic temperature management (TTM) such as pharmacological treatment, surface cooling devices with or without a feedback loop, and endovascular cooling devices during the acute phase of aSAH is uncertain.[37][255][256] It is reasonable to consider achieving normothermia during active or ongoing brain injury, while considering the consequences of TTM. TTM can be associated with complications such as shivering requiring pharmacologic control. Shivering should be monitored and managed to limit risk of secondary injury.[37][257]
Over 50% of survivors report cognitive impairment (e.g., mood and memory problems), resulting in a negative impact on functional status, emotional health, and quality of life.[160][161] Depression can occur in about one-third of aSAH survivors; anxiety and post-traumatic stress disorder can be seen in 15% to 20% of patients.[258][259][260] Use of validated grading scores or patient-reported outcome measures prior to hospital discharge is recommended to screen for physical, cognitive, behavioral, and quality of life deficits.[37] Use of validated screening tools in the postacute period is recommended to identify cognitive dysfunction; it is reasonable to choose the Montreal Cognitive Assessment (MoCA) over the Mini-Mental Status Examination (MMSE) to identify cognitive impairment.[37] In patients with depression, psychotherapy and pharmacotherapy are recommended to reduce symptoms.[37]
In-hospital mortality is around 20%, with all-cause mortality increasing to 25% at 6 months after SAH. In one study, 35% of patients were “dead or disabled” (defined as modified Rankin Scale ≥4) at 6 months.[159]
Chronic hydrocephalus is a well-known complication after aneurysmal SAH, occurring in >20% of patients.[184] In patients with aSAH and associated chronic symptomatic hydrocephalus, permanent CSF diversion is recommended to improve neurologic outcome.[37] Routine fenestration of the lamina terminalis is not indicated for reducing the rate of shunt dependency.[37][183]
Use of this content is subject to our disclaimer