Treatment is indicated if menopausal symptoms interfere with a woman's daily functioning and quality of life. The prevailing symptoms should be clarified, and lifestyle changes and drug therapy options (with benefits and risks) explained.
Hormone therapy should be given at the lowest dose and for the shortest time possible, while retaining the benefit of symptom relief. Since duration of symptoms cannot be predicted, there is no predetermined maximum duration of hormone therapy; all patients require individualized decision making. Reassessment should occur at least annually.
Management of hot flashes
Hot flashes can be treated with pharmacologic (hormonal and nonhormonal) or nonpharmacologic (lifestyle and alternative) approaches, or a combination of both.
Lifestyle changes
Despite limited evidence, certain lifestyle changes can improve symptom tolerance. Women should be encouraged to lose weight (where appropriate) and to exercise more.[11]Maas AHEM, Rosano G, Cifkova R, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J. 2021 Mar 7;42(10):967-84.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7947184
http://www.ncbi.nlm.nih.gov/pubmed/33495787?tool=bestpractice.com
Both have cardiovascular and bone health benefits and can improve overall wellbeing, but may not have a specifically identifiable impact on hot flashes and cannot improve bone density.[8]North American Menopause Society. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause. 2021 Sep 1;28(9):973-97.
http://www.ncbi.nlm.nih.gov/pubmed/34448749?tool=bestpractice.com
Other self-care measures include avoidance of spicy foods, alcohol, caffeine, warm environments, and stress. Alcohol and caffeine intake are associated with worsening vasomotor symptoms (VMS). Wearing layered clothing and use of hand-held fans, drinking cold water, and mist bottles may be helpful. One qualitative review of randomized controlled trials (RCTs) and meta-analyses found that yoga is moderately effective in the short term for psychological symptoms, but has no impact on somatic, vasomotor, or urogenital symptoms.[37]Cramer H, Lauche R, Langhorst J, et al. Effectiveness of yoga for menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012 Nov 7;2012:863905.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3524799
http://www.ncbi.nlm.nih.gov/pubmed/23304220?tool=bestpractice.com
Oral combination estrogen/progestin therapy
Hormonal therapy for a woman with a uterus comprises an estrogen, and a progestin to protect against endometrial hyperplasia and cancer.[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
Estrogens are the most effective treatment for VMS, reducing hot flashes by 80% to 90%.[38]American College of Obstetricians and Gynecologists. Practice bulletin no. 141: management of menopausal symptoms. Jan 2014 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2014/01/management-of-menopausal-symptoms
Combination regimens include:
The continuous combined regimen is indicated in women who have had amenorrhea for more than 12 months. It is easy to follow and continues the amenorrhea of menopause. If the woman has breakthrough bleeding after the first 6 months on the continuous combined regimen, endometrial assessment (pelvic ultrasound ± endometrial biopsy) is required and consideration should be given to switching to a sequential regimen so that she can have a predictable bleeding pattern.
In a sequential regimen, a progestin is added to an estrogen for the last 10 to 14 days of the cycle. Sequential hormone therapy (HT) may be preferred by perimenopausal women, but may also be indicated in postmenopausal women.[36]Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015 Nov;100(11):3975-4011.
https://academic.oup.com/jcem/article/100/11/3975/2836060
http://www.ncbi.nlm.nih.gov/pubmed/26444994?tool=bestpractice.com
[39]Hamoda H, Panay N, Peddar H, et al. The British Menopause Society & Women’s Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. 2020 [internet publication].
https://thebms.org.uk/publications/consensus-statements/bms-whcs-2020-recommendations-on-hormone-replacement-therapy-in-menopausal-women
Hormone therapy with an estrogen alone
Women without a uterus can take an estrogen alone if they do not have contraindications for systemic estrogen therapy.[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
[  ]
What are the benefits and harms of long-term estrogen-only hormone replacement therapy for healthy postmenopausal women?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1746/fullShow me the answer
]
What are the benefits and harms of long-term estrogen-only hormone replacement therapy for healthy postmenopausal women?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1746/fullShow me the answer
Transdermal administration of an estrogen
A transdermal formulation of estrogen (usually estradiol) is preferable to an oral estrogen in women who have a higher thrombotic risk (including body mass index >30), are taking other medications, have borderline triglyceride levels, are at risk for gallstones, or have difficulty adhering to a daily pill-taking regimen. Because there is no first-pass effect, a transdermal estrogen formulation may reduce the risk of thromboembolism compared with an oral estrogen, but this has not been studied in an RCT.[22]Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019 Jan 9;364:k4810.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6326068
http://www.ncbi.nlm.nih.gov/pubmed/30626577?tool=bestpractice.com
[23]Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause - 2017 update. Endocr Pract. 2017 Jul;23(7):869-80.
http://journals.aace.com/doi/full/10.4158/EP171828.PS
http://www.ncbi.nlm.nih.gov/pubmed/28703650?tool=bestpractice.com
It might also have a lower incidence of nausea. Long-term complications of transdermal estrogen formulations are probably the same as those of oral estrogens, although these were not specifically studied in the Women's Health Initiative. According to UK National Institute for Health and Care Excellence recommendations, a transdermal estrogen formulation at standard therapeutic doses does not increase the risk of venous thromboembolism compared with baseline population risk, nor is it likely to increase the risk of stroke.
Transdermal estradiol is available in patch, metered-dose spray, or gel formulations, all of which are applied to the skin. One 12-week, multicenter RCT found that, compared with placebo, transdermal estradiol gel significantly reduced the frequency and severity of hot flashes at weeks 4 and 12 in healthy menopausal women who had moderate to severe hot flashes.[40]Archer DF, Pickar JH, MacAllister DC, et al. Transdermal estradiol gel for the treatment of symptomatic postmenopausal women. Menopause. 2012 Jun;19(6):622-9.
http://www.ncbi.nlm.nih.gov/pubmed/22282101?tool=bestpractice.com
Headache, infection, breast pain, nausea, and insomnia were the most frequently reported side effects. A network meta-analysis found no difference in efficacy between an estradiol transdermal patch and an estradiol transdermal spray for vasomotor symptoms.[41]Kovács G, Zelei T, Vokó Z. Comparison of efficacy and local tolerability of estradiol metered-dose transdermal spray to estradiol patch in a network meta-analysis. Climacteric. 2016 Oct;19(5):488-95.
http://www.ncbi.nlm.nih.gov/pubmed/27593417?tool=bestpractice.com
Women with an intact uterus require protection with a progestin. A combination of an estrogen and a progestin is available in a single transdermal patch. Transdermal combined sequential HT preparations are also available.
Stopping estrogen therapy
There is insufficient evidence to recommend one method of stopping estrogen therapy over another. There is no standard age for discontinuation of HT. Each individual should be assessed on a regular basis (e.g., annually) to determine the extent of benefit from HT. For women who derive minimal benefit from HT, a reasonable discussion of treatment cessation may take place at any age.
Conjugated estrogens with bazedoxifene
Conjugated estrogens/bazedoxifene (a selective estrogen receptor modulator) has been approved by the Food and Drug Administration for the treatment of VMS and osteoporosis prevention in women with an intact uterus.[8]North American Menopause Society. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause. 2021 Sep 1;28(9):973-97.
http://www.ncbi.nlm.nih.gov/pubmed/34448749?tool=bestpractice.com
[42]Tella SH, Gallagher JC. Bazedoxifene + conjugated estrogens in HT for the prevention of osteoporosis and treatment of vasomotor symptoms associated with the menopause. Expert Opin Pharmacother. 2013 Dec;14(17):2407-20.
http://www.ncbi.nlm.nih.gov/pubmed/24093499?tool=bestpractice.com
Compared with conjugated estrogens/medroxyprogesterone, women treated with conjugated estrogens/bazedoxifene experienced significantly fewer adverse events. Women taking conjugated estrogens/bazedoxifene should not take a progestin.
Bazedoxifene has a favorable (anti-estrogenic) effect on breast tissue.[43]Valera MC, Gourdy P, Trémollières F, et al. From the Women's Health Initiative to the combination of estrogen and selective estrogen receptor modulators to avoid progestin addition. Maturitas. 2015 Nov;82(3):274-7.
http://www.ncbi.nlm.nih.gov/pubmed/26261036?tool=bestpractice.com
Further research is required.
Bioidentical hormones
Bioidentical hormone therapy (BHT) preparations are simply an estrogen (with or without a progestin) in a custom compounded base. Unregulated compounded BHT has been touted as "natural" and safer than conventional HT, but these claims are unsubstantiated.
One systematic review concluded that BHT is more effective than placebo for treating moderate to severe menopausal hot flashes (low to moderate quality evidence), but with higher rates of adverse effects.[44]Gaudard AM, Silva de Souza S, Puga ME, et al. Bioidentical hormones for women with vasomotor symptoms. Cochrane Database Syst Rev. 2016 Aug 1;(8):CD010407.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010407.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27479272?tool=bestpractice.com
There is no long-term safety data relating to outcomes such as myocardial infarction, stroke, and breast cancer.[44]Gaudard AM, Silva de Souza S, Puga ME, et al. Bioidentical hormones for women with vasomotor symptoms. Cochrane Database Syst Rev. 2016 Aug 1;(8):CD010407.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010407.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27479272?tool=bestpractice.com
Advisory bodies such as the North American Menopause Society, the American College of Obstetricians and Gynecologists, the US National Academies of Sciences, Engineering, and Medicine, and the British Menopause Society do not generally recommend compounded estrogen/progestin therapy due to the lack of standardized purity and potency with the attendant risks of over- or under-dosing.[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
[39]Hamoda H, Panay N, Peddar H, et al. The British Menopause Society & Women’s Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. 2020 [internet publication].
https://thebms.org.uk/publications/consensus-statements/bms-whcs-2020-recommendations-on-hormone-replacement-therapy-in-menopausal-women
[45]American College of Obstetricians and Gynecologists. ACOG clinical consensus no. 6: compounded bioidentical menopausal hormone therapy. Nov 2023 [internet publication].
https://www.acog.org/clinical/clinical-guidance/clinical-consensus/articles/2023/11/compounded-bioidentical-menopausal-hormone-therapy
[46]Stuenkel CA, Manson JE. Compounded bioidentical hormone therapy: the national academies weigh in. JAMA Intern Med. 2021 Mar 1;181(3):370-71.
http://www.ncbi.nlm.nih.gov/pubmed/33315055?tool=bestpractice.com
Similarly, the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) do not recommend BHT due to the lack of proven benefit and the potential for poor quality control.[23]Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause - 2017 update. Endocr Pract. 2017 Jul;23(7):869-80.
http://journals.aace.com/doi/full/10.4158/EP171828.PS
http://www.ncbi.nlm.nih.gov/pubmed/28703650?tool=bestpractice.com
For most women, licensed (regulated) HT provides appropriate therapy without the risks of custom preparations.
Regulated preparations containing estradiol and micronized progesterone (an estrogen similar to, and a progestogen equivalent to, those produced by the ovaries) should be used in preference to compounded preparations by women considering more natural preparations. The 2017 AACE/ACE position statement recommends that when the use of progesterone is necessary, micronized progesterone is considered the safer alternative to a synthetic progestin.[23]Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause - 2017 update. Endocr Pract. 2017 Jul;23(7):869-80.
http://journals.aace.com/doi/full/10.4158/EP171828.PS
http://www.ncbi.nlm.nih.gov/pubmed/28703650?tool=bestpractice.com
Micronized progesterone may be taken by the oral or vaginal route, but transdermal micronized progesterone does not protect the endometrium.[47]Stute P, Neulen J, Wildt L. The impact of micronized progesterone on the endometrium: a systematic review. Climacteric. 2016 Aug;19(4):316-28.
https://www.tandfonline.com/doi/full/10.1080/13697137.2016.1187123
http://www.ncbi.nlm.nih.gov/pubmed/27277331?tool=bestpractice.com
Bioidentical progesterone cream is available over the counter, but only one of three published RCTs showed some efficacy compared with placebo in reducing VMS.[48]Whelan AM, Jurgens TM, Trinacty M. Bioidentical progesterone cream for menopause-related vasomotor symptoms: is it effective? Ann Pharmacother. 2013 Jan;47(1):112-6.
http://www.ncbi.nlm.nih.gov/pubmed/23249728?tool=bestpractice.com
Risks of hormone therapy
It is important to provide information on the benefits and risks of HT, to help women make an informed choice about which, if any, treatment to use for menopausal symptoms.
[  ]
What are the benefits and harms of long-term combined hormone replacement therapy for healthy postmenopausal women?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1745/fullShow me the answer
[
]
What are the benefits and harms of long-term combined hormone replacement therapy for healthy postmenopausal women?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1745/fullShow me the answer
[  ]
What are the benefits and harms of long-term estrogen-only hormone replacement therapy for healthy postmenopausal women?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1746/fullShow me the answer
]
What are the benefits and harms of long-term estrogen-only hormone replacement therapy for healthy postmenopausal women?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1746/fullShow me the answer
Risk associated with HT use varies between women depending upon dose, duration, route of administration, age at initiation of therapy, and whether a progestogen is included in the regimen. The benefit-risk ratio of HT appears favorable for the management of VMS and the prevention of bone loss or fracture among women (without contraindications) who are under 60 years of age or are within 10 years of the menopause onset.[8]North American Menopause Society. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause. 2021 Sep 1;28(9):973-97.
http://www.ncbi.nlm.nih.gov/pubmed/34448749?tool=bestpractice.com
[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
During 18 years of follow-up, all-cause mortality among the pooled cohort of postmenopausal women who received 5 to 7 years of HT in the two Women's Health Initiative trials did not differ between the HT and placebo groups (27.1% versus 27.6%, respectively; hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.94 to 1.03]).[49]Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women's health initiative randomized trials. JAMA. 2017 Sep 12;318(10):927-38.
http://www.ncbi.nlm.nih.gov/pubmed/28898378?tool=bestpractice.com
Neither estrogen alone (HR 0.94, 95% CI 0.88 to 1.01) nor estrogen plus progestin (HR 1.02, 95% CI 0.96 to 1.08) were associated with increased risk of all-cause mortality.[49]Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women's health initiative randomized trials. JAMA. 2017 Sep 12;318(10):927-38.
http://www.ncbi.nlm.nih.gov/pubmed/28898378?tool=bestpractice.com
Heart disease, stroke, and venous thromboembolism
HT is not currently recommended for the primary prevention of cardiovascular disease.[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
[18]Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017 Jan 17;(1):CD004143.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004143.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/28093732?tool=bestpractice.com
[19]American College of Obstetricians and Gynecologists. ACOG committee opinion no. 565: hormone therapy and heart disease. Jun 2013 [internet publication].
https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2013/06/hormone-therapy-and-heart-disease
http://www.ncbi.nlm.nih.gov/pubmed/23812486?tool=bestpractice.com
[20]Kremer C, Gdovinova Z, Bejot Y, et al. European Stroke Organisation guidelines on stroke in women: Management of menopause, pregnancy and postpartum. Eur Stroke J. 2022 Jun;7(2):I-XIX.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9134774
http://www.ncbi.nlm.nih.gov/pubmed/35647308?tool=bestpractice.com
Further research is required to evaluate the impact of timing of HT initiation on coronary heart disease risk and mortality.
[Figure caption and citation for the preceding image starts]: Absolute rates of coronary heart disease for different types of hormone therapy (HT) compared with no HT (or placebo), different durations of HT use, and time since stopping HT for menopausal women.National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [Citation ends].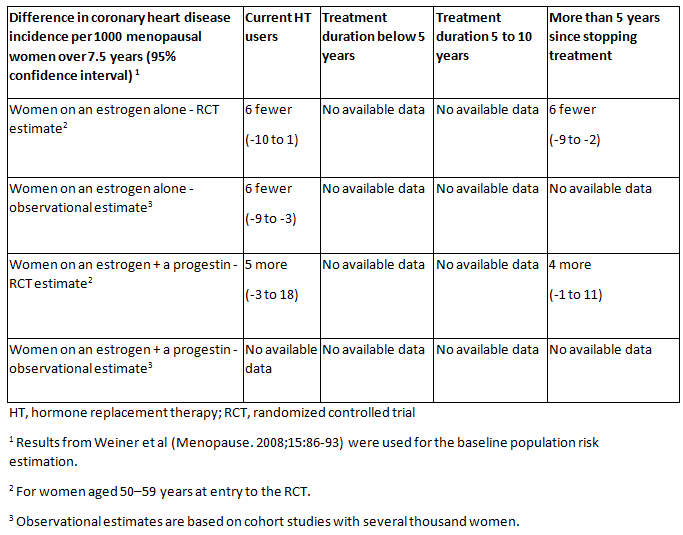
The risk of venous thromboembolism and ischemic stroke increases with oral HT, but the absolute risk of stroke in women under 60 years of age is very low.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
[50]de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013 Apr;16(2):203-4.
http://www.tandfonline.com/doi/full/10.3109/13697137.2013.771520
http://www.ncbi.nlm.nih.gov/pubmed/23488524?tool=bestpractice.com
[51]de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric. 2016 Aug;19(4):313-5.
http://www.ncbi.nlm.nih.gov/pubmed/27322027?tool=bestpractice.com
Observational studies and a meta-analysis indicate that transdermal estrogens are associated with a lower risk of venous thromboembolism and stroke than oral estrogens.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
[50]de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013 Apr;16(2):203-4.
http://www.tandfonline.com/doi/full/10.3109/13697137.2013.771520
http://www.ncbi.nlm.nih.gov/pubmed/23488524?tool=bestpractice.com
[51]de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric. 2016 Aug;19(4):313-5.
http://www.ncbi.nlm.nih.gov/pubmed/27322027?tool=bestpractice.com
Patients at high risk for cardiovascular complications should have a careful assessment of the risks and benefits of HT, and may be candidates for a trial of non-HT.[11]Maas AHEM, Rosano G, Cifkova R, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J. 2021 Mar 7;42(10):967-84.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7947184
http://www.ncbi.nlm.nih.gov/pubmed/33495787?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Absolute rates of stroke for different types of hormone therapy (HT) compared with no HT (or placebo), different durations of HT use, and time since stopping HT for menopausal women.National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [Citation ends].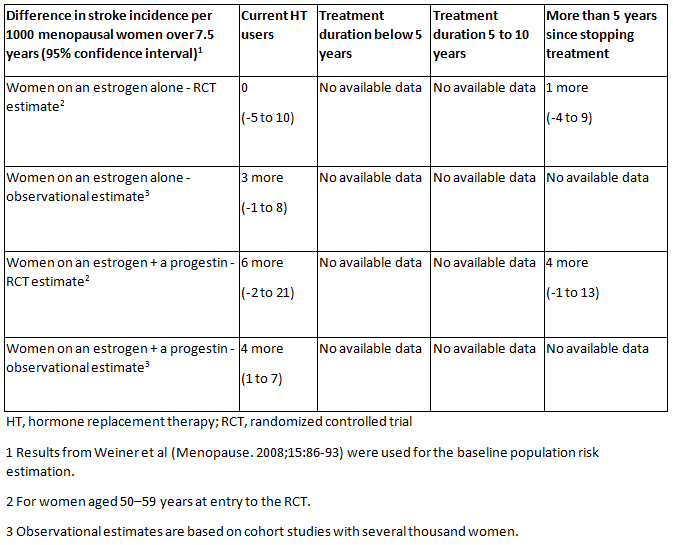
Breast and ovarian cancer
HT with an estrogen alone is associated with little or no change in the risk of breast cancer.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
An estrogen prescribed in combination with a progestin is associated with a small increase in the risk of breast cancer.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
[50]de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013 Apr;16(2):203-4.
http://www.tandfonline.com/doi/full/10.3109/13697137.2013.771520
http://www.ncbi.nlm.nih.gov/pubmed/23488524?tool=bestpractice.com
[51]de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric. 2016 Aug;19(4):313-5.
http://www.ncbi.nlm.nih.gov/pubmed/27322027?tool=bestpractice.com
[Evidence C]d5844f5f-2a2c-4a97-8747-d84790140572guidelineCWhat are the effects of hormone replacement therapy (HRT) administered for menopausal symptoms on the risk of developing breast cancer?[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
The increased risk is related to duration of treatment, and likely recedes after treatment is stopped.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
[50]de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013 Apr;16(2):203-4.
http://www.tandfonline.com/doi/full/10.3109/13697137.2013.771520
http://www.ncbi.nlm.nih.gov/pubmed/23488524?tool=bestpractice.com
[51]de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric. 2016 Aug;19(4):313-5.
http://www.ncbi.nlm.nih.gov/pubmed/27322027?tool=bestpractice.com
The UK Medicines and Healthcare products Regulatory Agency has published a table summarizing the risk of breast cancer for women currently receiving hormone therapy and post treatment from age of menopause up to age 69 years.[52]Medicines and Healthcare products Regulatory Agency. Drug safety update. Hormone replacement therapy (HRT): further information on the known increased risk of breast cancer with HRT and its persistence after stopping. Aug 2019 [internet publication].
https://www.gov.uk/drug-safety-update/hormone-replacement-therapy-hrt-further-information-on-the-known-increased-risk-of-breast-cancer-with-hrt-and-its-persistence-after-stopping
[Figure caption and citation for the preceding image starts]: Summary of HRT risks and benefits* during current use and current use plus post-treatment from age of menopause up to age 69 years, per 1000 women with 5 years or 10 years use of HRT. Key: *Menopausal symptom relief is not included in this table, but is a key benefit of HRT and will play a major part in the decision to prescribe HRT. †Best estimates based on relative risks of HRT use from age 50. For breast cancer this includes cases diagnosed during current HRT use and diagnosed after HRT use until age 69 years; for other risks, this assumes no residual effects after stopping HRT use. § Latest evidence suggests that transdermal HRT products have a lower risk of VTE than oral preparationsMedicines and Healthcare products Regulatory Agency. Hormone replacement therapy (HRT): further information on the known increased risk of breast cancer with HRT and its persistence after stopping. Aug 2019 [internet publication]; used with permission [Citation ends].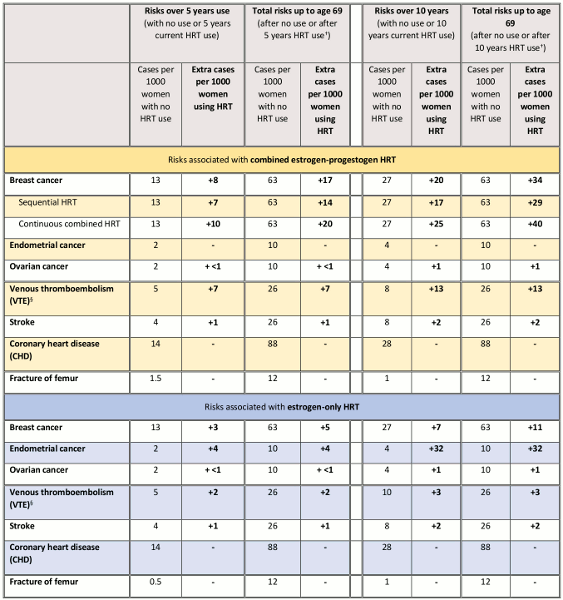
There is evidence to suggest that HT increases breast tissue density.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
This can make tumor detection more difficult, and may result in some women being recalled for repeat mammography and/or further evaluation.
A meta-analysis of 52 epidemiologic studies analyzed the risks of ovarian cancer in 12,110 postmenopausal women, 55% of whom had used HT for some period of time.[53]Beral V, Gaitskell K, Hermon C, et al.; Collaborative Group On Epidemiological Studies Of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015 May 9;385(9980):1835-42.
http://www.ncbi.nlm.nih.gov/pubmed/25684585?tool=bestpractice.com
The meta-analysis suggested that 5 years of HT use, starting at age 50 years, would result in one extra ovarian cancer per 1000 users.[53]Beral V, Gaitskell K, Hermon C, et al.; Collaborative Group On Epidemiological Studies Of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015 May 9;385(9980):1835-42.
http://www.ncbi.nlm.nih.gov/pubmed/25684585?tool=bestpractice.com
One 2019 meta-analysis of prospective studies found HT use for more than 1 year in postmenopausal women to be associated with an increased risk of breast cancer.[54]Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019 Aug 29;394(10204):1159-68.
www.doi.org/10.1016/S0140-6736(19)31709-X
http://www.ncbi.nlm.nih.gov/pubmed/31474332?tool=bestpractice.com
Risk increased with longer duration of HT use, and persisted after HT was stopped. Risk was higher with combined estrogen-progestogen compared with estrogen-only preparations, but there was no increased risk with topical vaginal estrogens. Current advice is unchanged - that HT should be used for the shortest time that it is needed, and patients should be vigilant for signs of breast cancer and encouraged to attend regular breast cancer screening.[52]Medicines and Healthcare products Regulatory Agency. Drug safety update. Hormone replacement therapy (HRT): further information on the known increased risk of breast cancer with HRT and its persistence after stopping. Aug 2019 [internet publication].
https://www.gov.uk/drug-safety-update/hormone-replacement-therapy-hrt-further-information-on-the-known-increased-risk-of-breast-cancer-with-hrt-and-its-persistence-after-stopping
[55]European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. Oct 2005 [internet publication].
https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-hormone-replacement-therapy-oestrogen-deficiency_en.pdf
The risks and benefits of HT should be discussed with patients during shared decision making before commencing HT.[1]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Dec 2019 [internet publication].
https://www.nice.org.uk/guidance/ng23
[56]British Menopause Society. BMS response to Lancet paper on the link between different forms of HRT and breast cancer incidence. Aug 2019 [internet publication].
https://thebms.org.uk/2019/08/bms-response-to-lancet-paper-on-the-link-between-different-forms-of-hrt-and-breast-cancer-incidence
Nonhormonal medications for vasomotor symptoms
A systematic review and meta-analysis of 43 trials of nonhormonal therapies found that estrogen replacement was significantly more effective than nonhormonal alternatives.[57]Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006 May 3;295(17):2057-71.
http://www.ncbi.nlm.nih.gov/pubmed/16670414?tool=bestpractice.com
However, nonhormonal alternatives may benefit women who are unable to take an estrogen because of risk factors or inability to tolerate HT.[58]The North American Menopause Society Advisory Panel. The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause. 2023 Jun 1;30(6):573-90.
http://www.ncbi.nlm.nih.gov/pubmed/37252752?tool=bestpractice.com
Selective serotonin-reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs)
SSRIs and SNRIs are effective for treating VMS in women unable to take HT.[59]Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011 Jan 19;305(3):267-74.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3129746
http://www.ncbi.nlm.nih.gov/pubmed/21245182?tool=bestpractice.com
[60]American Academy of Family Physicians. ACOG releases clinical guidelines on management of menopausal symptoms. Am Fam Physician. 2014 Sep 1;90(5):338-40.
https://www.aafp.org/afp/2014/0901/p338.pdf
[61]Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014 Jan;29(1):204-13.
http://www.ncbi.nlm.nih.gov/pubmed/23888328?tool=bestpractice.com
Some evidence suggests that escitalopram may be more effective than other SSRIs at reducing hot flashes.[61]Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014 Jan;29(1):204-13.
http://www.ncbi.nlm.nih.gov/pubmed/23888328?tool=bestpractice.com
Only paroxetine is approved for the treatment of moderate to severe VMS associated with menopause.[38]American College of Obstetricians and Gynecologists. Practice bulletin no. 141: management of menopausal symptoms. Jan 2014 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2014/01/management-of-menopausal-symptoms
[62]Orleans RJ, Li L, Kim MJ, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med. 2014 May 8;370(19):1777-9.
http://www.nejm.org/doi/full/10.1056/NEJMp1402080
http://www.ncbi.nlm.nih.gov/pubmed/24806158?tool=bestpractice.com
In an 8-week RCT of 339 perimenopausal and postmenopausal women with bothersome VMS, venlafaxine (an SNRI) reduced the frequency of symptoms by 1.8 more per day than placebo (P=0.005).[63]Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014 Jul;174(7):1058-66.
http://archinte.jamanetwork.com/article.aspx?articleid=1876676
http://www.ncbi.nlm.nih.gov/pubmed/24861828?tool=bestpractice.com
Low-dose estradiol appeared to be more effective (2.3 fewer VMS per day than placebo), but it was not compared directly with venlafaxine in this study.[63]Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014 Jul;174(7):1058-66.
http://archinte.jamanetwork.com/article.aspx?articleid=1876676
http://www.ncbi.nlm.nih.gov/pubmed/24861828?tool=bestpractice.com
Venlafaxine may be a reasonable alternative for women unable to take an estrogen.
Compared with placebo, desvenlafaxine (an SNRI) reduced the frequency and severity of moderate to severe hot flashes over a 12-month period in an RCT of 365 postmenopausal women with bothersome VMS.[64]Pinkerton JV, Archer DF, Guico-Pabia CJ, et al. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013 Jan;20(1):38-46.
http://www.ncbi.nlm.nih.gov/pubmed/23266839?tool=bestpractice.com
Subsequent to concerns regarding the safety of desvenlafaxine in this patient population, a discrete safety analysis found no evidence for an increased risk of cardiovascular, cerebrovascular, or hepatic events associated with desvenlafaxine (compared with placebo) for the treatment of menopausal VMS.[65]Archer DF, Pinkerton JV, Guico-Pabia CJ, et al. Cardiovascular, cerebrovascular, and hepatic safety of desvenlafaxine for 1 year in women with vasomotor symptoms associated with menopause. Menopause. 2013 Jan;20(1):47-56.
http://www.ncbi.nlm.nih.gov/pubmed/23266840?tool=bestpractice.com
Gabapentin
Studies indicate that gabapentin is moderately effective for the treatment of hot flashes.[36]Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015 Nov;100(11):3975-4011.
https://academic.oup.com/jcem/article/100/11/3975/2836060
http://www.ncbi.nlm.nih.gov/pubmed/26444994?tool=bestpractice.com
[66]Hayes LP, Carroll DG, Kelley KW. Use of gabapentin for the management of natural or surgical menopausal hot flashes. Ann Pharmacother. 2011 Mar;45(3):388-94.
http://www.ncbi.nlm.nih.gov/pubmed/21343402?tool=bestpractice.com
However, drowsiness, dizziness, and unsteadiness are commonly reported adverse effects.[66]Hayes LP, Carroll DG, Kelley KW. Use of gabapentin for the management of natural or surgical menopausal hot flashes. Ann Pharmacother. 2011 Mar;45(3):388-94.
http://www.ncbi.nlm.nih.gov/pubmed/21343402?tool=bestpractice.com
Gabapentin may result in intolerable lethargy when used during the day.[36]Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015 Nov;100(11):3975-4011.
https://academic.oup.com/jcem/article/100/11/3975/2836060
http://www.ncbi.nlm.nih.gov/pubmed/26444994?tool=bestpractice.com
Side effects can be mitigated by dosing only at night, or by using a dose escalation regimen.
Clonidine
Clonidine, an antihypertensive agent, reduces hot flashes but may be less effective than SSRIs/SNRIs and gabapentin.[36]Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015 Nov;100(11):3975-4011.
https://academic.oup.com/jcem/article/100/11/3975/2836060
http://www.ncbi.nlm.nih.gov/pubmed/26444994?tool=bestpractice.com
Hypotension may be a treatment-limiting adverse effect.[67]Goldberg RM, Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994 Jan;12(1):155-8.
http://www.ncbi.nlm.nih.gov/pubmed/8270972?tool=bestpractice.com
Blood pressure should be monitored during therapy and for rebound after discontinuation. Transdermal patches result in stable blood levels and are preferred to oral clonidine preparations.[36]Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015 Nov;100(11):3975-4011.
https://academic.oup.com/jcem/article/100/11/3975/2836060
http://www.ncbi.nlm.nih.gov/pubmed/26444994?tool=bestpractice.com
Symptoms of urogenital atrophy
Low-dose vaginal estrogen preparations can be considered in women with symptoms of urogenital atrophy.[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
A review of Medline and Cochrane databases showed that vaginal estrogens are more effective than placebo for improving dryness and decreasing dyspareunia, urinary urgency, and urinary frequency.[68]Rahn DD, Carberry C, Sanses TV, et al.; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014 Dec;124(6):1147-56.
http://www.ncbi.nlm.nih.gov/pubmed/25415166?tool=bestpractice.com
Rates of urinary tract infections also declined with vaginal estrogen use. Serum estradiol levels remained within postmenopausal norms, except for those who used high-dose vaginal estrogen cream.[68]Rahn DD, Carberry C, Sanses TV, et al.; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014 Dec;124(6):1147-56.
http://www.ncbi.nlm.nih.gov/pubmed/25415166?tool=bestpractice.com
In women without a history of hormone-dependent cancer, vaginal estrogens can be continued for symptom relief, and there is no evidence to recommend endometrial surveillance.[31]North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013 Sep;20(9):888-902.
http://www.menopause.org/docs/default-source/2013/vva-position-statement.pdf?sfvrsn=0
http://www.ncbi.nlm.nih.gov/pubmed/23985562?tool=bestpractice.com
In the Women's Health Initiative observational study, the risk of cardiovascular disease, invasive breast cancer, and endometrial cancer did not differ between women who were vaginal estrogen users and those who were not.[69]Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018 Jan;25(1):11-20.
http://www.ncbi.nlm.nih.gov/pubmed/28816933?tool=bestpractice.com
Vaginal estrogens do not require progestin replacement.
Vaginal estradiol tablets do not result in significant systemic absorption and can be used long-term as required. However, in October 2019, the European Medicines Agency recommended limiting the use of high-strength estradiol vaginal creams (containing estradiol 100 micrograms/g or 0.01%) to a single treatment period of up to 4 weeks. This is because levels of estradiol in the blood were found to be higher than normal postmenopausal levels and could result in similar adverse effects to those seen with systemic (oral or transdermal) HT (e.g., venous thromboembolism, stroke, endometrial cancer, breast cancer). This formulation should not be used in patients already on HT.[70]European Medicines Agency. Four-week limit for use of high-strength estradiol creams. Press release. Oct 2019 [internet publication].
https://www.ema.europa.eu/en/news/four-week-limit-use-high-strength-estradiol-creams
Therefore, other vaginal estrogen formulations (e.g., conjugated estrogen cream, estradiol intravaginal tablets and rings) may be preferred. There is no evidence to suggest that any one vaginal estrogen preparation is more efficacious than another.[71]Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016 Aug 31;(8):CD001500.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001500.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27577677?tool=bestpractice.com
Ospemifene is a selective estrogen receptor modulator indicated for the treatment of dyspareunia in postmenopausal women. In a phase 3 trial, ospemifene increased the percentage of superficial cells and reduced dyspareunia compared with placebo.[72]Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013 Jun;20(6):623-30.
http://www.ncbi.nlm.nih.gov/pubmed/23361170?tool=bestpractice.com
Hot flashes were the most frequently reported adverse event (ospemifene 7% versus placebo 4%).[72]Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013 Jun;20(6):623-30.
http://www.ncbi.nlm.nih.gov/pubmed/23361170?tool=bestpractice.com
[73]Palacios S, Cancelo MJ. Clinical update on the use of ospemifene in the treatment of severe symptomatic vulvar and vaginal atrophy. Int J Womens Health. 2016 Oct 26;8:617-26.
https://www.dovepress.com/clinical-update-on-the-use-of-ospemifene-in-the-treatment-of-severe-sy-peer-reviewed-fulltext-article-IJWH
http://www.ncbi.nlm.nih.gov/pubmed/27822125?tool=bestpractice.com
Breast and gynecologic cancer survivors
Consensus recommendations for the management of menopausal symptoms after breast cancer include:[74]Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017 Oct 1;102(10):3647-61.
http://www.ncbi.nlm.nih.gov/pubmed/28934376?tool=bestpractice.com
[75]American College of Obstetricians and Gynecologists. Clinical consensus no. 2: treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer. Dec 2021 [internet publication].
https://www.acog.org/clinical/clinical-guidance/clinical-consensus/articles/2021/12/treatment-of-urogenital-symptoms-in-individuals-with-a-history-of-estrogen-dependent-breast-cancer
Generally avoiding treatment with systemic menopausal hormone therapy or vaginal estrogen*
Implementing lifestyle measures (e.g., healthy diet, regular physical activity, smoking cessation, weight loss, limiting or avoiding alcohol, maintaining adequate levels of vitamin D and calcium)
Nonhormonal, pharmacologic therapy (e.g., selective serotonin/noradrenaline reuptake inhibitors), and/or cognitive behavioral therapy.
*There are data to suggest that HT does not increase risk of recurrence in women who do not have an estrogen-dependent malignancy.[76]Del Carmen MG, Rice LW. Management of menopausal symptoms in women with gynecologic cancers. Gynecol Oncol. 2017 Aug;146(2):427-35.
http://www.ncbi.nlm.nih.gov/pubmed/28625396?tool=bestpractice.com
[77]O'Donnell RL, Clement KM, Edmondson RJ. Hormone replacement therapy after treatment for a gynaecological malignancy. Curr Opin Obstet Gynecol. 2016 Feb;28(1):32-41.
http://www.ncbi.nlm.nih.gov/pubmed/26626038?tool=bestpractice.com
One review suggested that short-term use of HT may result in improvement of menopausal vasomotor and genitourinary symptoms in patients with gynecologic cancer who do not have an estrogen-dependent malignancy.[76]Del Carmen MG, Rice LW. Management of menopausal symptoms in women with gynecologic cancers. Gynecol Oncol. 2017 Aug;146(2):427-35.
http://www.ncbi.nlm.nih.gov/pubmed/28625396?tool=bestpractice.com
The use of estrogens in women with non-estrogen-dependent malignancy must be tailored to the patient's situation.
Polycarbophil gel, a vaginal moisturizer, can also be offered as an effective treatment for symptoms of vaginal atrophy, including dryness and dyspareunia.[78]Goetsch MF, Lim JY, Caughey AB. Locating pain in breast cancer survivors experiencing dyspareunia: a randomized controlled trial. Obstet Gynecol. 2014 Jun;123(6):1231-6.
http://www.ncbi.nlm.nih.gov/pubmed/24807329?tool=bestpractice.com
[79]Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014 Sep;36(9):830-3.
https://www.jogc.com/article/S1701-2163(15)30487-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25222364?tool=bestpractice.com
Dyspareunia is common in breast cancer survivors due to atrophy and dryness from lack of estrogen. In a small trial, lidocaine reduced tenderness in the vulvar vestibule of postmenopausal breast cancer survivors with dyspareunia (median coital pain score 8 on a 10-point scale) compared with normal saline.[78]Goetsch MF, Lim JY, Caughey AB. Locating pain in breast cancer survivors experiencing dyspareunia: a randomized controlled trial. Obstet Gynecol. 2014 Jun;123(6):1231-6.
http://www.ncbi.nlm.nih.gov/pubmed/24807329?tool=bestpractice.com
Intravaginal dehydroepiandrosterone (DHEA) and oral ospemifene are approved for the management of dyspareunia, but safety following breast cancer has not been established.[74]Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017 Oct 1;102(10):3647-61.
http://www.ncbi.nlm.nih.gov/pubmed/28934376?tool=bestpractice.com
Reduced libido
Women with distressing low sexual desire and tiredness should be counseled that androgen supplementation is an option, particularly if an estrogen with or without a progestin has not been effective.
The Endocrine Society recommends against diagnosing androgen deficiency in healthy menopausal women because there is a lack of a well-defined syndrome and data correlating androgen levels with specific signs or symptoms are not available.[80]Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014 Oct;99(10):3489-510.
https://academic.oup.com/jcem/article/99/10/3489/2836272
http://www.ncbi.nlm.nih.gov/pubmed/25279570?tool=bestpractice.com
See Sexual dysfunction in women.
Urinary stress incontinence
Pelvic floor rehabilitation may be useful for urinary stress incontinence.[81]Dumoulin C, Cacciari LP, Hay‐Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018 Oct 4;(10):CD005654.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005654.pub4/full?highlightAbstract=floor%7Cpelvic
In one study, 73.4% of postmenopausal women randomized to a combination of intravaginal estriol and pelvic floor rehabilitation for 6 months experienced subjective improvement in stress urinary incontinence compared with only 9.71% of women in the control (estriol-only) group.[82]Capobianco G, Donolo E, Borghero G, et al. Effects of intravaginal estriol and pelvic floor rehabilitation on urogenital aging in postmenopausal women. Arch Gynecol Obstet. 2012 Feb;285(2):397-403.
http://www.ncbi.nlm.nih.gov/pubmed/21706345?tool=bestpractice.com
Both groups reported improvement in signs and symptoms of urogenital atrophy.
Sleep disturbance and mood symptoms
Difficulty sleeping or nocturnal restlessness and awakening are common problems during menopause. Insomnia is often associated with hot flashes (other factors, such as depression or sleep apnea, may also be involved). But no studies have shown a direct physiologic link.
Some women report improved sleep while taking HT.[17]The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022 Jul 1;29(7):767-94.
http://www.ncbi.nlm.nih.gov/pubmed/35797481?tool=bestpractice.com
[83]Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005 May;23(2):117-25.
http://www.ncbi.nlm.nih.gov/pubmed/15852197?tool=bestpractice.com
In one systematic review of 11 studies of varying quality, progesterone improved VMS and sleep quality.[84]Spark MJ, Willis J. Systematic review of progesterone use by midlife and menopausal women. Maturitas. 2012 Jul;72(3):192-202.
http://www.ncbi.nlm.nih.gov/pubmed/22541358?tool=bestpractice.com
Data suggest that short-term use of HT may improve mood and depressive symptoms during the menopausal transition and in the early menopause, but study results are inconsistent.[85]Gordon JL, Girdler SS. Hormone replacement therapy in the treatment of perimenopausal depression. Curr Psychiatry Rep. 2014 Dec;16(12):517.
http://www.ncbi.nlm.nih.gov/pubmed/25308388?tool=bestpractice.com
[86]Veerus P, Hovi SL, Sevón T, et al. The effect of hormone therapy on women's quality of life in the first year of the Estonian Postmenopausal Hormone Therapy trial. BMC Res Notes. 2012 Apr 3;5:176.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3349465
http://www.ncbi.nlm.nih.gov/pubmed/22472039?tool=bestpractice.com
There is evidence to suggest that cognitive behavioral therapy (CBT) may have a beneficial role in the management of low mood and anxiety in women with menopausal symptoms after breast cancer treatment.[87]Mann E, Smith MJ, Hellier J, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 2012 Mar;13(3):309-18.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3314999
http://www.ncbi.nlm.nih.gov/pubmed/22340966?tool=bestpractice.com
There is a lack of good quality evidence relating to CBT specifically in women with depression related to early menopause but given the low risk of harms and strong evidence base for treating depression in the general population it is a reasonable option in women who are perimenopausal with depression.[88]Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018 Oct;25(10):1069-85.
https://journals.lww.com/menopausejournal/Fulltext/2018/10000/Guidelines_for_the_evaluation_and_treatment_of.5.aspx
http://www.ncbi.nlm.nih.gov/pubmed/30179986?tool=bestpractice.com
While HT or other alternatives may improve sleeping patterns, an assessment should be made for other underlying factors that may require targeted treatment. Mood disorders, notably depression, often improve with HT, although conventional antidepressants may be more effective.[89]Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med. 2005 Dec 19;118(suppl 12B):93-7.
http://www.ncbi.nlm.nih.gov/pubmed/16414333?tool=bestpractice.com
Women with symptoms of severe depression should be referred for mental health assessment.
Alternative or herbal therapies
A Cochrane review of 43 RCTs involving phytoestrogens found that there was a large placebo effect (from 1% to 59%) in most of the trials.[90]Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013 Dec 10;(12):CD001395.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001395.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/24323914?tool=bestpractice.com
This, and other reviews, have found no conclusive evidence that phytoestrogens, including isoflavones, are effective for the management of menopausal VMS.[38]American College of Obstetricians and Gynecologists. Practice bulletin no. 141: management of menopausal symptoms. Jan 2014 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2014/01/management-of-menopausal-symptoms
[90]Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013 Dec 10;(12):CD001395.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001395.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/24323914?tool=bestpractice.com
[91]Sacks FM, Lichtenstein A, Van Horn L, et al; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006 Feb 21;113(7):1034-44.
http://circ.ahajournals.org/content/113/7/1034.long
http://www.ncbi.nlm.nih.gov/pubmed/16418439?tool=bestpractice.com
Acupuncture and reflexology have not been shown to significantly improve VMS compared with placebo.[38]American College of Obstetricians and Gynecologists. Practice bulletin no. 141: management of menopausal symptoms. Jan 2014 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2014/01/management-of-menopausal-symptoms
 ]
]
 ]
[
]
[  ]
]


