Lesch-Nyhan disease (LND) should be considered when delayed development is accompanied by a hyperkinetic disorder, particularly when brain MRI is normal.
It should be suspected if delayed development is accompanied by self-injurious behavior or evidence of excessive production of uric acid. A clinical suspicion should always be confirmed by hypoxanthine-guanine phosphoribosyltransferase (HPRT) molecular gene analysis, and preferably also HPRT enzyme activity.
Virtually all patients are male, owing to the X-linked recessive mode of inheritance. However, a few female patients have been described.[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570. A history of LND in other family members might point toward the diagnosis. LND has been reported in most ethnic groups, with approximately equal rates.
Clinical features
Classic LND patients usually come to clinical attention before the age of 1 year.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
Most patients come to medical attention early in life, usually before 4 years of age. People with an LN variant (LNV) might present at a later age, depending on the age of onset of renal or neurologic problems.[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570.
Typically, self-injurious behavior starts at age 2 to 5 years, although cases of around 18 years of age at onset have been described.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
Finger and lip biting is a frequently seen form of self-injurious behavior. Subsequent partial amputations of the fingers, lips, tongue, and oral mucosa are common. Such topographic preference is rarely seen in other diseases with self-injury.
Among the most frequent presenting symptoms in classic LND is a failure to reach motor milestones.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
Sometimes previously achieved motor milestones are lost. Cognitive function is usually impaired, with average intelligence quotient values of approximately 70, although normal intelligence has been described in some patients. Patients do not have global intellectual disability, but rather have impairments in specific cognitive domains involving attention and mental flexibility. Involuntary movements are common among the presenting symptoms, although they may develop later in the course of the disease.[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570. A generalized action dystonia is present, characterized by frequent extraneous movements in the face, neck, and limbs, with sustained muscle contractions. This results in twisted postures that interfere with voluntary movement.[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
On examination, somatic growth is affected more than head circumference or bone age.[23]Watts RW, Harkness RA, Spellacy E, et al. Lesch-Nyhan syndrome: growth delay, testicular atrophy and a partial failure of the 11 beta-hydroxylation of steroids. J Inherit Metab Dis. 1987;10(3):210-23.
http://www.ncbi.nlm.nih.gov/pubmed/2828760?tool=bestpractice.com
[24]Mizuno T. Long-term follow-up of ten patients with Lesch-Nyhan syndrome. Neuropediatrics. 1986 Aug;17(3):158-61.
http://www.ncbi.nlm.nih.gov/pubmed/3762872?tool=bestpractice.com
[25]Christie R, Bay C, Kaufman IA, et al. Lesch-Nyhan disease: clinical experience with nineteen patients. Dev Med Child Neurol. 1982 Jun;24(3):293-306.
http://www.ncbi.nlm.nih.gov/pubmed/7095300?tool=bestpractice.com
A generalized hypotonia is frequently seen at presentation.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
Spasticity and hyperreflexia, implying the involvement of corticospinal pathways, may be present;[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570. these features usually appear later in the course of the disease, and are often asymmetrical. The cause is unknown, but they may be a result of myelopathy resulting from forceful involuntary movements of the neck.[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570. The presence of orange "sand" crystals in the diaper should be checked or asked about if it is not reported spontaneously. The "sand" and orange color is caused by uric acid crystals and microhematuria.[24]Mizuno T. Long-term follow-up of ten patients with Lesch-Nyhan syndrome. Neuropediatrics. 1986 Aug;17(3):158-61.
http://www.ncbi.nlm.nih.gov/pubmed/3762872?tool=bestpractice.com
[25]Christie R, Bay C, Kaufman IA, et al. Lesch-Nyhan disease: clinical experience with nineteen patients. Dev Med Child Neurol. 1982 Jun;24(3):293-306.
http://www.ncbi.nlm.nih.gov/pubmed/7095300?tool=bestpractice.com
Testicular atrophy is commonly seen, and puberty is often delayed or absent.[23]Watts RW, Harkness RA, Spellacy E, et al. Lesch-Nyhan syndrome: growth delay, testicular atrophy and a partial failure of the 11 beta-hydroxylation of steroids. J Inherit Metab Dis. 1987;10(3):210-23.
http://www.ncbi.nlm.nih.gov/pubmed/2828760?tool=bestpractice.com
Undescended testes also occur.[23]Watts RW, Harkness RA, Spellacy E, et al. Lesch-Nyhan syndrome: growth delay, testicular atrophy and a partial failure of the 11 beta-hydroxylation of steroids. J Inherit Metab Dis. 1987;10(3):210-23.
http://www.ncbi.nlm.nih.gov/pubmed/2828760?tool=bestpractice.com
[24]Mizuno T. Long-term follow-up of ten patients with Lesch-Nyhan syndrome. Neuropediatrics. 1986 Aug;17(3):158-61.
http://www.ncbi.nlm.nih.gov/pubmed/3762872?tool=bestpractice.com
[26]Watts RW, Spellacy E, Gibbs DA, et al. Clinical, post-mortem, biochemical and therapeutic observations on the Lesch-Nyhan syndrome with particular reference to the neurological manifestations. Q J Med. 1982;51(201):43-78.
http://www.ncbi.nlm.nih.gov/pubmed/7111674?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Examples of self-injurious behavior seen in patients with classic Lesch-Nyhan diseaseFrom the collection of H.A. Jinnah, MD, PhD; used with permission [Citation ends].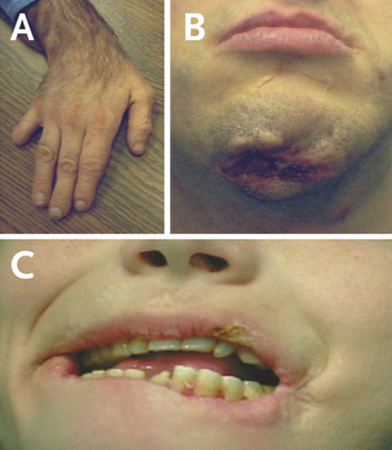
Hyperuricemia
The majority of LND patients have elevated uric acid levels in serum and urine as a result of HPRT deficiency.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
Uric acid levels are frequently evaluated as part of the metabolic workup of developmental delay or hypotonia. Urine is best evaluated by 24-hour urinary uric acid-creatinine ratio, or a total 24-hour uric acid excretion.[13]Becker MA. Hyperuricemia and gout. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2513-2535. Although high uric acid levels may provide important clues to the diagnosis, they lack sufficient sensitivity and specificity for definitive diagnosis.
The hyperuricemia is also associated with nephrolithiasis, gouty arthritis, and subcutaneous tophi.[Figure caption and citation for the preceding image starts]: Uric acid levels in patients with classic Lesch-Nyhan disease (LND), patients with Lesch-Nyhan variants (LNV), and healthy controls. SD, standard deviation; HRH: hypoxanthine-guanine phosphoribosyltransferase (HPRT)-related hyperuricemia; HRD: HPRT-related neurologic diseaseFrom the collection of J.E. Visser, MD, PhD and H.A. Jinnah, MD, PhD; used with permission [Citation ends].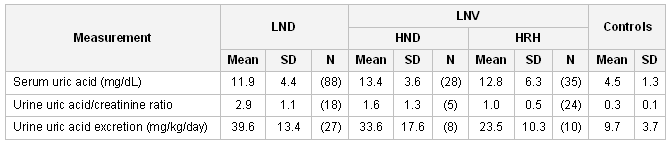
HPRT enzyme activity
Measurements of HPRT activity in cultured intact cells, such as fibroblasts, are considered more accurate than those in cell lysates.[3]Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill; 2001:2537-2570. The percentage of residual HPRT activity can provide some predictive value concerning disease severity. The clinical phenotype is a continuum:[27]Hersh JH, Page T, Hand ME, et al. Clinical correlations in partial hypoxanthine guanine phosphoribosyltransferase deficiency. Pediatr Neurol. 1986 Sep-Oct;2(5):302-4.
http://www.ncbi.nlm.nih.gov/pubmed/3508703?tool=bestpractice.com
[28]Page T, Bakay B, Nissinen E. Hypoxanthine-guanine phosphoribosyltransferase variants: correlation of clinical phenotype with enzyme activity. J Inherit Metab Dis. 1981;4(4):203-6.
http://www.ncbi.nlm.nih.gov/pubmed/6796771?tool=bestpractice.com
[29]Page T, Nyhan WL. The spectrum of HPRT deficiency: an update. Adv Exp Med Biol. 1989;253A:129-33.
http://www.ncbi.nlm.nih.gov/pubmed/2624181?tool=bestpractice.com
Near complete absence of HPRT activity results in the full phenotype of classic LND
A residual activity of ≥1.5% usually prevents self-injury and other behavioral disturbances
Residual activity >8% rarely causes obvious neurologic impairment.
Laboratories where HPRT activity can be measured are listed on the website of the Lesch-Nyhan Disease International Study Group.
Lesch-Nyhan disease international study group
Opens in new window
HPRT gene analysis
Rapid and reliable tests have been developed to identify HPRT gene mutations using molecular genetic methods. The mutations are heterogeneous, including point mutations and other substitutions, deletions, and insertions.[11]Jinnah HA, De Gregorio L, Harris JC, et al. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000 Oct;463(3):309-26.
http://www.ncbi.nlm.nih.gov/pubmed/11018746?tool=bestpractice.com
[12]Fu R, Ceballos-Picot I, Torres RJ, et al; Lesch-Nyhan Disease International Study Group. Genotype-phenotype correlations in neurogenetics: Lesch-Nyhan disease as a model disorder. Brain. 2014 May;137(Pt 5):1282-303.
http://brain.oxfordjournals.org/content/137/5/1282.long
http://www.ncbi.nlm.nih.gov/pubmed/23975452?tool=bestpractice.com
Mutations that predict large aberrations in the resulting protein, such as large deletions or early nonsense mutations, or a mutation identified in a prior patient, appear to be good predictors of disease severity.[11]Jinnah HA, De Gregorio L, Harris JC, et al. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000 Oct;463(3):309-26.
http://www.ncbi.nlm.nih.gov/pubmed/11018746?tool=bestpractice.com
[12]Fu R, Ceballos-Picot I, Torres RJ, et al; Lesch-Nyhan Disease International Study Group. Genotype-phenotype correlations in neurogenetics: Lesch-Nyhan disease as a model disorder. Brain. 2014 May;137(Pt 5):1282-303.
http://brain.oxfordjournals.org/content/137/5/1282.long
http://www.ncbi.nlm.nih.gov/pubmed/23975452?tool=bestpractice.com
Point mutations may cause either classic LND or an LNV, depending on the ultimate effect on enzyme activity.
In the US, molecular samples can be sent to Emory Genetics Laboratory.
Emory genetics laboratory
Opens in new window Other laboratories are listed on the website of the Lesch-Nyhan Disease International Study Group.
Lesch-Nyhan disease international study group
Opens in new window
Brain imaging
Brain imaging is not normally necessary for diagnosis and management of Lesch-Nyhan disease, but may be helpful if there is clinical suspicion of other diagnoses. Generally, neither CT scanning nor MRI reveal any obvious structural malformations or signal changes.[22]Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006 May;129(Pt 5):1201-17.
https://academic.oup.com/brain/article/129/5/1201/327116/Delineation-of-the-motor-disorder-of-Lesch-Nyhan
http://www.ncbi.nlm.nih.gov/pubmed/16549399?tool=bestpractice.com
[30]Ernst M, Zametkin AJ, Matochik JA, et al. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med. 1996 Jun 13;334(24):1568-72.
http://www.nejm.org/doi/full/10.1056/NEJM199606133342403#t=article
http://www.ncbi.nlm.nih.gov/pubmed/8628337?tool=bestpractice.com
Routine imaging is usually normal, but may reveal mild loss of brain volume.[31]Harris JC, Lee RR, Jinnah HA, et al. Craniocerebral magnetic resonance imaging measurement and findings in Lesch-Nyhan syndrome. Arch Neurol. 1998 Apr;55(4):547-53.
http://archneur.jamanetwork.com/article.aspx?articleid=773664
http://www.ncbi.nlm.nih.gov/pubmed/9561984?tool=bestpractice.com

