Approach
MGUS is typically asymptomatic, and a monoclonal (M) protein is often detected incidentally in the serum or urine. By definition, the diagnosis of MGUS requires the absence of anemia, hypercalcemia, renal failure, and lytic bone lesions related to plasma cell proliferative disorders.
History and clinical features
Family history of hematologic malignancies (including MGUS or multiple myeloma), male sex, age >50 years, African ancestry, and exposure to radiation or pesticides are associated with a higher prevalence of MGUS.[5][6][7][8][9][18] A personal history of autoimmunity (such as rheumatoid arthritis, systemic lupus erythematosus, or Sjogren syndrome), immunosuppressive medications, and/or repeated infections should be sought because of possible associations with MGUS.[8][15]
Although almost all patients with MGUS are asymptomatic, those with IgM MGUS in particular may present with symptoms of peripheral neuropathy. Such patients may present with typical sensory symptoms such as paresthesia, dysesthesia, or neuropathic pain associated with ataxia and gait disturbance. On investigation, they may lack specific autoantibodies to confirm the causal association between the monoclonal gammopathy and the polyneuropathy. It is thus important to consider the possibility of other polyneuropathies, such as chronic inflammatory demyelinating polyneuropathy and paraneoplastic, metabolic, and toxic neuropathies, which may coexist with an M protein, and arrange for appropriate management. Amyloidosis should also be considered as an alternative diagnosis. Any chronic or intermittent bone pain should be evaluated because it is commonly associated with myeloma.
Hepatosplenomegaly, lymphadenopathy, chronic or intermittent bone pain, weight loss, diarrhea, dysphagia, edema, dyspnea, dizziness, bleeding and bruising, and night sweats or fever, may be indicative of progression to another disorder, such as lymphoma, chronic lymphocytic leukemia, multiple myeloma, or amyloidosis.
Tests
The presence of MGUS is suspected in an asymptomatic patient with M protein detected in the serum at a concentration <3 g/dL. Electrophoresis and immunofixation are used to identify and measure M protein in the serum.
To differentiate MGUS from a related plasma cell malignancy, patients should have a complete blood count, serum creatinine, serum calcium, and a complete radiographic bone survey of the skeleton, including all long bones (e.g., using conventional x-ray or whole body low-dose computed tomography scan). Patients who have abnormalities should have additional tests to determine whether the abnormalities are related to a plasma cell proliferative disorder. By definition, the diagnosis of MGUS requires the absence of anemia, hypercalcemia, renal failure, and lytic bone lesions related to the plasma cell proliferative disorder. Serum monoclonal (M) protein concentration >3 g/dL by definition indicates multiple myeloma or a related malignancy, but some of these patients have smoldering/asymptomatic myeloma and remain stable during long-term follow up.[27] Only patients in whom these clinical findings of end-organ damage are felt to be clearly related to the plasma cell disorder are considered to have multiple myeloma. Although the predictive value may not be high, higher concentrations of serum M proteins are associated with a greater likelihood of malignancy.[28]
Other tests for evaluating MGUS in an asymptomatic patient include:
Routine urinalysis and 24-hour urine collection with electrophoresis and immunofixation: dipstick testing of the urine for protein is not sufficient; it primarily detects albumin, but it often does not detect immunoglobulin light chains.
Serum free light chains assay: measures free circulating kappa and lambda light chains in the blood. An abnormal free light chain ratio indicates that the proportion of free circulating kappa and lambda light chains is abnormally high or low (equivalent to too much or too little kappa or lambda). An abnormal ratio is an adverse risk factor for multiple myeloma progression.[29]
Bone marrow aspiration and/or biopsy should be considered if the M protein is >1.5 g/dL, or for non-IgG MGUS, or if the free light chain ratio is abnormal. A bone marrow exam is also needed in patients who have unexplained anemia or other cytopenias, renal failure, hypercalcemia, or bone lesions on a metastatic bone survey. The probability of marrow having ≥10% plasma cells can be estimated using the iStopMM calculator, helping to predict the need for bone marrow sampling. iStopMM: predicting the need for bone marrow sampling in MGUS Opens in new window
Imaging of the spine and pelvis using magnetic resonance imaging or positron emission tomography may be used to evaluate bone pathology, and to help predict the likelihood of progression to myeloma. Dual energy x-ray absorptiometry (DEXA) imaging may be used to assess bone mineral density, as patients with MGUS have an increased risk of osteoporosis.[Figure caption and citation for the preceding image starts]: Using electrophoresis and immunofixation, figure A shows normal results while figure B shows evidence of an IgA kappa MGUS with polyclonal IgG backgroundFrom the collection of Ola Landgren, MD, PhD, courtesy of Dr Jerry Katzmann, Mayo Clinic, Rochester, MN [Citation ends].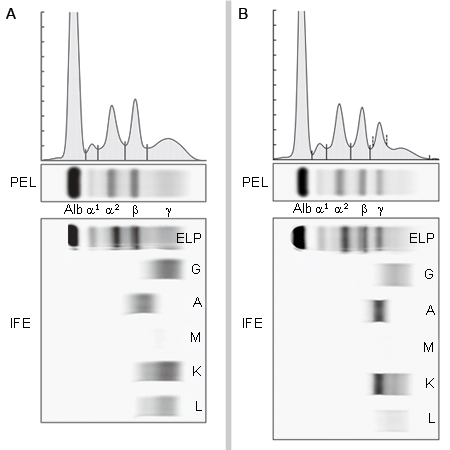 [Figure caption and citation for the preceding image starts]: Hematoxylin-eosin section of bone marrow biopsy showing mild increase in plasma cellsFrom the collection of Ola Landgren, MD, PhD [Citation ends].
[Figure caption and citation for the preceding image starts]: Hematoxylin-eosin section of bone marrow biopsy showing mild increase in plasma cellsFrom the collection of Ola Landgren, MD, PhD [Citation ends].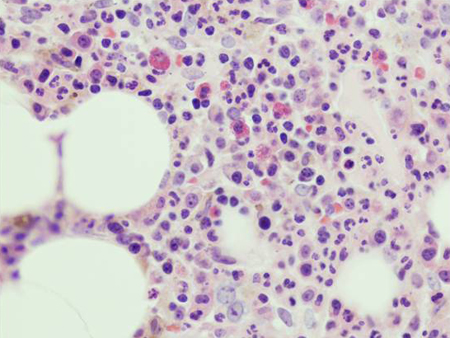 [Figure caption and citation for the preceding image starts]: CD138 immunohistochemical staining highlighting small clusters of plasma cellsFrom the collection of Ola Landgren, MD, PhD [Citation ends].
[Figure caption and citation for the preceding image starts]: CD138 immunohistochemical staining highlighting small clusters of plasma cellsFrom the collection of Ola Landgren, MD, PhD [Citation ends].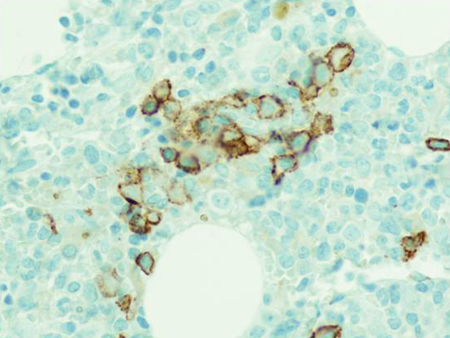
Use of this content is subject to our disclaimer