Approach
Postnatal rubella is most commonly diagnosed by serologic testing in patients who present with a generalized maculopapular rash, fever >99°F (37.2°C), and arthralgia/arthritis, lymphadenopathy, or conjunctivitis. Diagnostic testing is indicated for people with risk factors for rubella (under-immunization, known contact with a case of rubella, travel to a region of the world where rubella is endemic) and a clinical picture consistent with rubella. Identifying contagious people may prevent the spread of rubella to susceptible contacts, especially pregnant women. Diagnostic testing is also indicated for people who have risk factors for rubella and who have potential complications of the disease, for example, arthritis, thrombocytopenia, or encephalitis.
Clinical features
Rash is often the first manifestation of rubella in young children. The rash of rubella is erythematous, discrete, maculopapular, and sometimes mildly pruritic, and may be accentuated by heat. It usually begins on the face and spreads from the head to the feet. Occasionally there may be a petechial component to the rash or palatal petechiae. The rash persists for an average of 3 to 4 days.
Low-grade fever of >99°F (37.2°C) occurs in up to 50% of infections. Prodromal malaise and other mild constitutional symptoms are more common in adults than in children. Mild upper respiratory symptoms are common in children of school age and adults, and may precede the onset of rash by several days. Arthralgias and arthritis are frequent in adults (occurring in up to 70% of adult women) but uncommon in children. The most common joints affected are the fingers, wrists, and knees. The onset of joint symptoms usually coincides with the rash, and symptoms may persist for weeks. Rarely, symptoms may be recurrent or chronic.[22]
Mild lymphadenopathy involving the postauricular, posterior cervical, and occipital lymph node groups occurs in almost all patients and may precede the onset of rash by up to 1 week. Nodes are typically nontender and mobile. Nonpurulent conjunctivitis is reported in about 70% of adolescents and adults, but is less common in children.[Figure caption and citation for the preceding image starts]: Rubella rashCenters for Disease Control and Prevention (CDC) Public Health Image Library [Citation ends].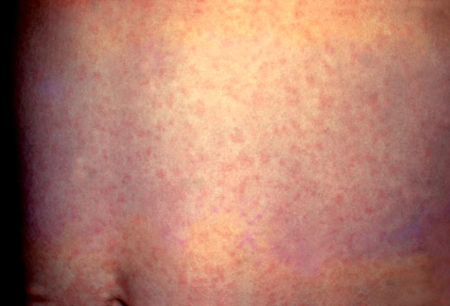
Tests
Antibody testing
Suspected cases of rubella should be confirmed by serologic testing. Anti-rubella IgM can be detected by enzyme immunoassay at the onset of clinical illness in almost all patients and persists for weeks to months. The optimum time-point for collection of serum is 5 days after the onset of symptoms (fever and rash), when >90% of cases will be IgM positive.[5] However, because rubella is a rare disease and the specificity of the test is <100%, false-positives are possible. Rubella IgM assays may produce false-positive results for several reasons, such as cross-reacting IgM resulting from infection with viruses other than rubella (such as parvovirus), the presence of rheumatoid factor, and persistent IgM after infection or vaccination.[23] A positive test, therefore, should always be confirmed by measuring anti-rubella IgG in paired acute and convalescent sera (drawn 2 to 3 weeks apart), or by measuring IgG avidity (the overall strength of binding between the antigen and antibody, which increases with time as the immune response matures). A 4-fold rise in serum rubella IgG or seroconversion between acute and convalescent samples indicates acute infection and is useful as a confirmatory test or if a false-negative rubella IgM is suspected. Low avidity IgG antibodies can be detected for up to 4 months after infection and indicate recent infection, while the presence of high avidity IgG suggests a more distant exposure (which may be from either infection or vaccination). Rubella IgG can last a lifetime. Detection of rubella IgG should be used for assessing rubella immunity, including before, during, and after pregnancy. CDC: laboratory protocols - rubella Opens in new window CDC: laboratory support for surveillance of vaccine-preventable diseases Opens in new window
Rubella IgM can be used to diagnose congenital rubella syndrome cases. Suspected cases should be tested as close to birth as possible, and again at 1 month of age if the initial IgM test is negative. If paired sera are to be collected, the second sample should be collected 14 to 21 days after the acute specimen was collected. At 3 months of age, approximately 50% of cases would still have detectable rubella IgM in their serum. The presence of rubella IgG in an infant after the decline of maternal antibodies (around 9 months of age) and the absence of vaccination or exposure to rubella will also confirm congenital rubella syndrome.[5]
Reverse transcription polymerase chain reaction (RT-PCR)
Detection of rubella RNA in direct clinical specimens or after incubation in tissue culture can also confirm infection. This is available commercially and through several state health departments. Nasopharyngeal swabs are the preferred sample type. The utility of RT-PCR is limited because of the narrow window when the virus can be detected in clinical samples; in respiratory samples, rubella RNA is typically only detectable from 2 days before rash onset to 4 days after. Swabs should be collected as soon after symptom onset as possible, preferably one to 3 days after onset, but no later than 7 days postonset.[5]
RT-PCR assays on throat swabs, nasopharyngeal swabs, and urine specimens from a neonate can be used for confirmation of suspected congenital rubella syndrome cases. Samples should be collected prior to 3 months of age if possible, because by 3 months of age approximately 50% will no longer shed virus.[5]
Viral culture
Rubella virus can be isolated from the nasopharynx, throat, urine, blood, and CSF from about 1 week before to 2 weeks after the onset of rash. Isolation of rubella virus from clinical specimens is diagnostic; however, viral cultures are not routinely obtained because they are labor-intensive and performed only in specialized reference laboratories. Molecular typing of rubella isolates by PCR is invaluable for epidemiologic purposes however, and viral isolation should be attempted in all cases of confirmed or strongly suspected rubella. Testing is especially important for pregnant women who may require expert maternal-fetal management due to the risk of congenital rubella syndrome.
CBC
Typically, no other routine labs are needed. A CBC may be obtained if patients develop petechiae due to thrombocytopenia or if other more serious infections are suspected.
Use of this content is subject to our disclaimer