History and exam
Key diagnostic factors
common
maternal risk factors for child with spina bifida
Prenatal history should focus on known risk factors for having a child with spina bifida, including poor maternal nutrition (inadequate folate and vitamin B12 intake), exposure to potential teratogens (e.g., valproic acid, carbamazepine, isotretinoin, or methotrexate), and presence of maternal diabetes and/or obesity.
history of elevated triple or quadruple screening test during prenatal assessment
Both tests are routinely offered between 15 and 20 weeks' gestation. An elevated level is suggestive of spina bifida.
history of abnormality on prenatal ultrasound
Prenatal ultrasound identifies a neural tube defect.
open spina bifida lesion: myelomeningocele, myeloschisis, meningocele
Lesions that are not covered by skin are termed open spina bifida. Types include the following.
Myelomeningocele: herniation of both meninges and spine. Associated with hydrocephalus and Chiari II malformation.
Myeloschisis: herniation of meninges and spinal elements. Characterized by flattened, plate-like mass of disorganized neural tissue.
Meningocele: herniation of the meninges without involvement of spinal elements. [Figure caption and citation for the preceding image starts]: Neonate with myelomeningoceleFrom the collection of Dr Greg Liptak; used with permission [Citation ends].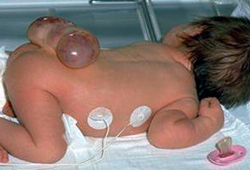 [Figure caption and citation for the preceding image starts]: MyelomeningoceleFrom the collection of Dr Greg Liptak; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: MyelomeningoceleFrom the collection of Dr Greg Liptak; used with permission [Citation ends].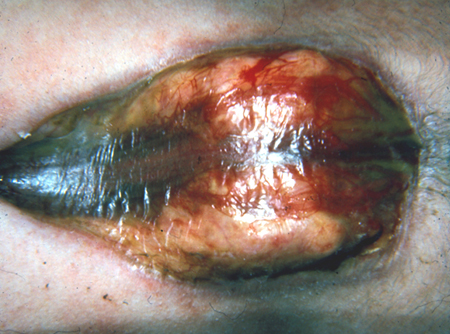 [Figure caption and citation for the preceding image starts]: MeningoceleFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: MeningoceleFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].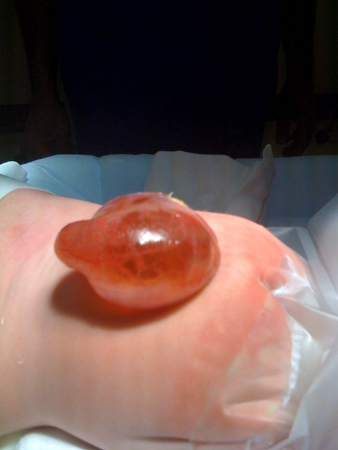
closed spina bifida lesion: asymmetric gluteal fold or dimple, hemangioma, hairy patch, or other cutaneous markings
Lesions that are covered by skin are termed closed spina bifida.
Neurocutaneous markings along the cervical spinal axis, such as hair whorls, may indicate underlying spine or vertebral anomalies such as syrinx, diastematomyelia, and hemivertebrae. [Figure caption and citation for the preceding image starts]: MyelomeningocystoceleFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].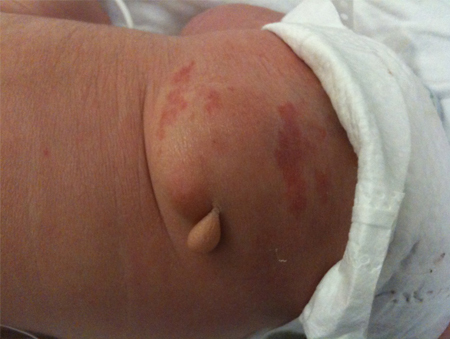 [Figure caption and citation for the preceding image starts]: Skin tagFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Skin tagFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].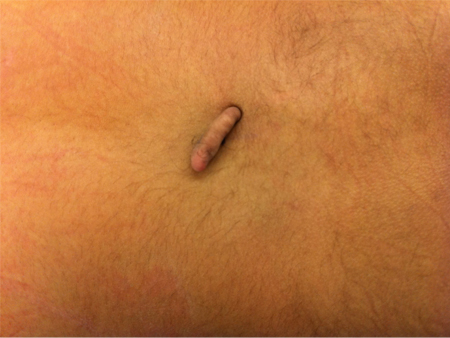
bulging fontanelle
A bulging or tense fontanelle is a common sign of increased intracranial pressure and hydrocephalus in an infant with spina bifida.
rapid head growth
Head growth crossing 2 percentile categories. Indicates hydrocephalus.
abnormal urinary voiding
Indicates neurogenic bladder. Infants should wet a minimum of 4 diapers in a 24-hour period. A distended bladder and decreased urine output suggests incomplete emptying. Constant urinary dribbling suggests an open bladder outlet, overflow incontinence, or an ectopic ureter.
leakage of meconium or stool
Suggests low anal sphincter tone and neurogenic bowel.
uncommon
midline congenital anomalies: cleft lip or palate, cardiac murmur
It is estimated that as many as 10% of fetuses with neural tube defects have associated chromosomal anomalies, particularly trisomy 18, trisomy 13, and 22q deletion syndrome (also known as velocardiofacial syndrome).
A genetic evaluation is warranted in any infant born with spina bifida who has other midline congenital anomalies.[55]
arching of neck
This is a worrisome sign and usually indicates pressure on the brainstem due to shunt malfunction and/or dysfunction intrinsic to the Chiari II malformation.
Requires prompt evaluation by a neurosurgeon.
Other diagnostic factors
common
absence of anal wink/rectal tone
Usually reflects the underlying deficit and is a sign of neurogenic bowel. May be a temporary (4-6 months) finding due to spinal shock from the myelomeningocele repair.
downward deviation of the eyes (sundowning)
Indicates hydrocephalus.
upward and lateral deviation of eyes
Caused by cranial nerve lesions from pressure on brainstem (Chiari malformation).
abnormal cry
Hydrocephalus may cause a stridulous cry.
Chiari malformations may cause a hoarse or high-pitched cry.
breathing abnormalities: apnea, inspiratory stridor, snoring
facial asymmetry
Caused by cranial nerve lesions from pressure on brainstem (Chiari malformation).
asymmetry of spontaneous arm and leg movement
May be noted by the parents. Parents may also report apparent pain in the limbs, muscle weakness, and atrophy.
difficulty with diapering or dressing
This may indicate decreased range of motion in the legs or the presence of spasticity.
abnormal muscle tone and bulk in arms and legs
Tone varies depending on level of lesion. For example, infants with L4 level paraplegia may be born with wasting of the gastrocnemius muscle group.
decreased sensation
Demonstrated by reduced or absent response to pinprick in the feet, legs, and buttocks.
hip subluxation or dislocation
May have asymmetric hip abduction, or positive Barlow or Ortolani sign (eliciting a clunk from the hip as it relocates).
Often does not cause functional change; therefore, surgery is only considered for low sacral-lesion ambulatory patients.
clubfoot (equinovarus deformity)
Clubfoot is a common deformity with lumbar or higher level lesions due to imbalance of muscles around the foot and ankle.
vertical talus deformity
Manifests as a rigid rocker-bottom flat foot. Reflects weakness in ankle plantar flexors.
hip and knee flexion contractures
May be apparent at birth.
uncommon
feeding difficulties
Feeding difficulties may be related to a malfunctioning shunt or due to intrinsic malformation of the brainstem (Chiari malformation).
congenital scoliosis
Congenital scoliosis is uncommon and does not typically require treatment in the newborn period or infancy. It is usually due to a large spine defect, which is an orthopedic issue. However, scoliosis beyond this period may indicate underlying spine or vertebral anomalies such as syrinx, diastematomyelia, and hemivertebrae, and warrants diagnostic neuroimaging.
Scoliosis eventually affects almost all adolescent and adult patients who have myelomeningoceles above the sacral level.[90]
congenital kyphosis
Usually located in the thoracic spine and can be severe. Surgery is rarely performed in the newborn period or infancy.
Risk factors
strong
inadequate maternal folate intake
Regarded as the single most clinically significant factor contributing to the incidence of spina bifida. The mechanism may be related to defects in folate metabolism (counteracted by supplemental folic acid) rather than malnutrition.[50][51] The relative risk of a neural tube defect in women with low folate levels is 2 to 8 times that of the general population.[32]
The US Preventive Services Task Force recommends that all women who are planning, or capable of, pregnancy take supplemental folic acid to prevent neural tube defects.[52]
See Primary prevention.
previous pregnancy affected by spina bifida or other neural tube defect
The recurrence rate of neural tube defects, for women who have had one previous affected pregnancy, is 3% to 4%.[7][8][9] If two previous pregnancies have been affected, the recurrence rate is 10% to 20%.[10][11]
This recurrence risk can be reduced substantially by high-dose folate supplementation.[53]
The Centers for Disease Control and Prevention and the US Preventive Services Task Force recommend that women at risk for recurrence and planning a pregnancy consult their physician about high-dose folate supplementation, commencing 1 month prior to pregnancy and continuing throughout the first trimester.[52][54]
See Primary prevention.
maternal history of spina bifida or other neural tube defect
Women who have spina bifida themselves have an increased risk of having a baby with spina bifida compared with the general population.[2][35] This risk can be reduced substantially by high-dose folate supplementation.[53]
The Centers for Disease Control and Prevention and the US Preventive Services Task Force recommend that women at risk for recurrence and planning a pregnancy consult their physician about high-dose folate supplementation, commencing 1 month prior to pregnancy and continuing throughout the first trimester.[52][54]
See Primary prevention.
Hispanic ancestry or ethnicity
Recent Hispanic immigrant parents from Mexico or Central America residing in the US have more than a 3-fold increased risk of having a pregnancy affected by a neural tube defect, compared with the general population. US-born Hispanic women have a 2-fold increased risk of having a pregnancy affected by anencephaly, whereas rates of spina bifida are similar to those for non-Hispanic white women.[33][34]
trisomy 18 or trisomy 13
prenatal exposure to valproic acid, carbamazepine, isotretinoin, or methotrexate
Maternal use of anticonvulsant medications, such as valproic acid or carbamazepine, has been associated with 10- to 20-fold excess risk of neural tube defects when taken during pregnancy.[36] In addition, lower IQ scores have been noted among children who were exposed to these medications prenatally, particularly valproic acid.[58] Isotretinoin and methotrexate are also known to increase the risk of pregnancies affected by neural tube defects.[37]
inadequate maternal vitamin B12 intake
A 3-fold excess risk of neural tube defects was documented in a population-based case-control study of Mexican-American women whose postpartum B12 levels were in the lowest quartile, compared with women with the highest quartile of postpartum B12 concentrations.[44]
maternal obesity
Body mass index >29 doubles the risk for neural tube defects. Severe obesity increases the risk more than 3-fold.[59]
weak
female sex of infant
Spina bifida is 1.2 to 1.7 times more common in girls, except for sacral-level defects, which occur with equal frequency among boys and girls.[13]
22q deletion syndrome
There have been case reports of co-occurrence of 22q deletion syndrome (also known as velocardiofacial syndrome) and spina bifida.
A genetic evaluation is warranted in any infant born with spina bifida who has other midline congenital anomalies, such as conotruncal heart defects or cleft lip and/or cleft palate.[55]
low socioeconomic status
Contributes to the risk of neural tube defects, even after adjusting for maternal nutritional status.[39]
elevated maternal core body temperature during first trimester
Maternal core body temperature elevated by 2⁰ F or C during the first trimester, either due to fever or to the use of saunas or hot tubs, has been shown to increase the risk of neural tube defects in pregnancy.[40]
Use of this content is subject to our disclaimer