Tests
1st tests to order
CBC with differential
Test
A first test to order if primary myelofibrosis (PMF) is suspected.
The presence of blood count abnormalities should prompt a careful exam of peripheral blood smear.
Because of its origin in a multipotent hematopoietic progenitor cell, PMF affects all blood cell types but not in a predictable manner.
Anemia, usually mild, is present in most patients with PMF, with >60% having a hemoglobin concentration <10 g/dL.
Leukocyte and platelet counts can be low, normal, or high without reference to spleen size. Thrombocytosis is more common than thrombocytopenia. Disseminated intravascular coagulation is observed in 15% of patients and can manifest as thrombocytopenia, decreased clotting factors, and increased fibrin degradation products.
Result
anemia; leukocyte and platelet count normal or abnormal
peripheral blood smear
Test
A first test to order if primary myelofibrosis (PMF) is suspected.
In patients with PMF, peripheral blood smear will usually show immature white cells (metamyelocytes, myelocytes, promyelocytes, myeloblasts), nucleated red blood cells (RBCs), and teardrop-shaped RBCs, as a result of extramedullary hematopoiesis. This leukoerythroblastic reaction is not specific for PMF, but it is a hallmark of PMF and its absence should challenge the clinical impression. [Figure caption and citation for the preceding image starts]: Peripheral blood smear showing teardrop red blood cells (black arrows), 2 nucleated red blood cells (red arrows), and a myelocyte (blue arrow)From the collection of A. Emadi and J.L. Spivak; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Peripheral blood smear showing leukoerythroblastic reaction: teardrop red blood cells (black arrows), myelocyte (red arrow), and promyelocyte (blue arrow)From the collection of A. Emadi and J.L. Spivak; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Peripheral blood smear showing leukoerythroblastic reaction: teardrop red blood cells (black arrows), myelocyte (red arrow), and promyelocyte (blue arrow)From the collection of A. Emadi and J.L. Spivak; used with permission [Citation ends].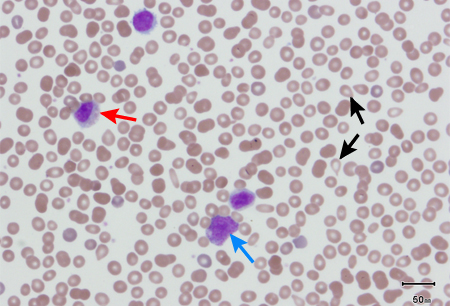
Result
presence of metamyelocytes, myelocytes, promyelocytes, myeloblasts; nucleated RBCs; and teardrop-shaped RBCs in the circulation
bone marrow aspiration
Test
Bone marrow aspiration and biopsy are essential for establishing a diagnosis.
Bone marrow fibrosis is a hallmark of primary myelofibrosis (PMF). Its presence is mandated for the diagnosis of the overt fibrotic stage of PMF.[38] See Criteria.
Bone marrow aspiration (with a properly placed biopsy needle) usually results in a "dry tap" in patients with PMF.
Result
unable to aspirate marrow ("dry tap")
bone marrow biopsy
Test
Bone marrow aspiration and biopsy are essential for establishing a diagnosis.
Bone marrow fibrosis is a hallmark of primary myelofibrosis (PMF). Its presence is mandated for the diagnosis of the overt fibrotic stage of PMF.[38] See Criteria.
Biopsy will typically reveal marrow fibrosis (reticulin fibrosis) and megakaryocytic proliferation and atypia.
Bone marrow cellularity may be increased (with trilineage hyperplasia and erythroblastic and megakaryocytic islands), or decreased (with scattered areas of hyperplastic marrow embedded in a collagenous matrix), or hypoplastic (with intense osteomyelosclerosis and residual megakaryocytic islands). [Figure caption and citation for the preceding image starts]: Bone marrow biopsy showing increased reticulin depositionFrom the collection of A. Emadi and J.L. Spivak; used with permission [Citation ends].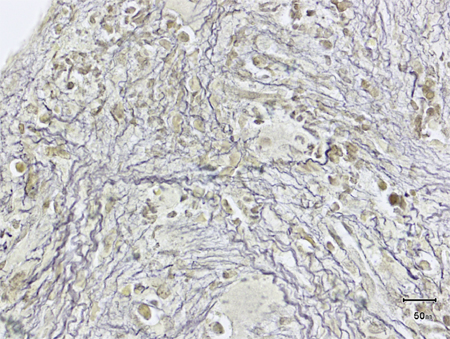 [Figure caption and citation for the preceding image starts]: Trephine bone marrow biopsy showing megakaryocytic hyperplasia and clusteringFrom the collection of A. Emadi and J.L. Spivak; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Trephine bone marrow biopsy showing megakaryocytic hyperplasia and clusteringFrom the collection of A. Emadi and J.L. Spivak; used with permission [Citation ends].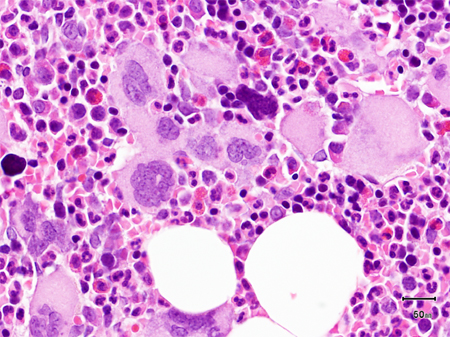
Bone marrow fibrosis alone is not sufficient to diagnose PMF because it can occur in other myeloproliferative neoplasms (e.g., polycythemia vera, essential thrombocythemia, chronic myeloid leukemia) and other hematologic disorders (e.g., systemic mastocytosis, hairy cell leukemia, myelodysplasia, primary marrow lymphoma, and acute leukemia). Distinguishing between PV/ET and early "prefibrotic" PMF may be difficult based on histological findings, but accurate diagnosis is essential to optimize management.[33]
Further cytogenetic and molecular testing is required to exclude other disorders that can cause myelofibrosis.
Result
marrow fibrosis; megakaryocytic proliferation and atypia; increase or decrease bone marrow cellularity
fluorescence in situ hybridization (FISH) or multiplex reverse transcriptase PCR
Test
For the presence of BCR::ABL1 fusion gene. Required to exclude chronic myeloid leukemia.
Result
negative for Philadelphia chromosome (BCR::ABL1 fusion gene)
genetic mutation analysis
Test
All patients with suspected PMF should undergo molecular testing for the JAK2 V617F mutation initially. If negative, testing for CALR and MPL mutations should follow.[26][39] Alternatively, a next-generation sequencing panel comprising all three MPN driver mutations can be used, which also provides quantitative assessment of driver mutation allele burden.
Presence of JAK2 V617F, MPL, or CALR mutation indicates a myeloproliferative neoplasm (MPN), but these mutations are not specific for primary myelofibrosis (PMF).
Incidence of JAK2 V617F, MPL, or CALR mutation in PMF patients is reported to be approximately 58%, 8%, and 25%, respectively.[14][15]
Approximately 10% of PMF patients are negative for JAK2, CALR, and MPL mutations (i.e., triple negative); therefore, absence of these MPN driver mutations does not exclude the diagnosis.[24] Some triple-negative patients may have uncommon MPL mutations.[25]
Although MPN driver mutations are not mutually exclusive, patients typically only have one driver mutation that is clonally dominant.
Testing for non-driver mutations (e.g., ASXL1, EZH2, SRSF2, U2AF1 Q157, IDH1/2, SF3B1, TET2) should be carried out for prognostication and risk stratification following diagnosis.[24]
Result
may be positive for MPN driver mutation (JAK2 V617F, CALR, or MPL) and/or non-driver mutations (e.g., ASXL1, EZH2, SRSF2, U2AF1 Q157, IDH1/2, SF3B1, TET2)
Tests to consider
bone marrow cytogenetic analysis
Test
Chromosomal abnormalities, e.g., involving chromosomes 13 (del.13q), 20 (del.20q), +8 (trisomy 8), 1, 5 (-5/del5q), 7 (-7/del7q), +9 (trisomy 9), 12 (del12p), and 17, are reported in approximately 45% of patients with primary myelofibrosis (PMF).[12]
Chromosomal abnormalities are not diagnostic for PMF, but their identification can facilitate risk stratification and prognostication.
Sole +9 (trisomy 9), 13 (del.13q), 20 (del.20q), and normal cytogenetics have the most favorable prognosis; sole +8 (trisomy 8), 5 (-5/del5q), 7 (-7/del7q), 12 (del12p), 17 (i(17q)), and complex mutations have a distinctly poorer prognosis.[10][11][12][13]
Result
may reveal cytogenetic abnormalities, e.g., involving chromosome 13 (del.13q), 20 (del.20q), +8 (trisomy 8), 1, 5 (-5/del5q), 7 (-7/del7q), +9 (trisomy 9), 12 (del12p), or 17
echocardiogram
Test
May be helpful in uncovering pulmonary hypertension, a manifestation of extramedullary hematopoiesis.
Imaging studies should not routinely be used unless extramedullary hematopoiesis is suspected and the site needs to be identified for treatment.
Result
enlarged main pulmonary artery
ultrasound of suspected site
Test
May be helpful in uncovering extramedullary hematopoiesis.
Imaging studies should not routinely be used unless extramedullary hematopoiesis is suspected and the site needs to be identified for treatment.
Result
evidence of extramedullary hematopoiesis
technetium 99 scan
Test
May be helpful in uncovering pulmonary extramedullary hematopoiesis.
Imaging studies should not routinely be used unless extramedullary hematopoiesis is suspected and the site needs to be identified for treatment.
Result
evidence of extramedullary hematopoiesis
CT of suspected site
Test
May be helpful in uncovering extramedullary hematopoiesis.
Imaging studies should not routinely be used unless extramedullary hematopoiesis is suspected and the site needs to be identified for treatment.
Result
evidence of extramedullary hematopoiesis
MRI of suspected site
Test
May be helpful in uncovering spinal extramedullary hematopoiesis.
Imaging studies should not routinely be used unless extramedullary hematopoiesis is suspected and the site needs to be identified for treatment.
Result
evidence of extramedullary hematopoiesis
serum uric acid
Test
Serum uric acid levels may be >7 mg/dL in men and >6 mg/dL in women in cases of hyperuricemia.
Hyperuricemia is a consequence of increased cell turnover and could cause kidney stones or gout, particularly with cytoreductive therapy.
Result
normal or elevated
antinuclear antibodies
Test
Autoimmune phenomena are characteristic of primary myelofibrosis (PMF).
Testing for autoreactivity can be carried out if clinically indicated (e.g., joint complaints, evidence of hemolysis, or unexplained thrombocytopenia).
Results may reveal circulating immune complexes; complement activation; elevated antinuclear antibody, elevated rheumatoid factor titers; and/or a positive Coombs test in the absence of an overt connective tissue disorder.
Result
may be elevated
rheumatoid factor titer
Test
Autoimmune phenomena are characteristic of primary myelofibrosis (PMF).
Testing for autoreactivity can be carried out if clinically indicated (e.g., joint complaints, evidence of hemolysis, or unexplained thrombocytopenia).
Results may reveal circulating immune complexes; complement activation; elevated antinuclear antibody, elevated rheumatoid factor titers; and/or a positive Coombs test in the absence of an overt connective tissue disorder.
Result
may be elevated
complement levels
Test
Autoimmune phenomena are characteristic of primary myelofibrosis (PMF).
Testing for autoreactivity can be carried out if clinically indicated (e.g., joint complaints, evidence of hemolysis, or unexplained thrombocytopenia).
Results may reveal circulating immune complexes; complement activation; elevated antinuclear antibody, elevated rheumatoid factor titers; and/or a positive Coombs test in the absence of an overt connective tissue disorder.
Result
may reveal complement activation
Coombs test
Test
Autoimmune phenomena are characteristic of primary myelofibrosis (PMF).
Testing for autoreactivity can be carried out if clinically indicated (e.g., joint complaints, evidence of hemolysis, or unexplained thrombocytopenia).
Results may reveal circulating immune complexes; complement activation; elevated antinuclear antibody, elevated rheumatoid factor titers; and/or a positive Coombs test.
PMF should be suspected with a positive Coombs test in the absence of an overt connective tissue disorder.
Result
may be positive
Use of this content is subject to our disclaimer