History and exam
Key diagnostic factors
common
boys: testes ≥4 mL
Testicular size is documented as a measurement of the longest axis or by the testicular volume using the Prader orchidometer. A volume of ≥4 mL, or a length of 2.5 cm (1 inch), defines the onset of puberty.[2]
[Figure caption and citation for the preceding image starts]: Prader orchidometerCreated by BMJ Knowledge Centre [Citation ends]. [Figure caption and citation for the preceding image starts]: Method of comparing testicular size using the Prader orchidometerFrom the collection of Dr A. Mehta; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Method of comparing testicular size using the Prader orchidometerFrom the collection of Dr A. Mehta; used with permission [Citation ends].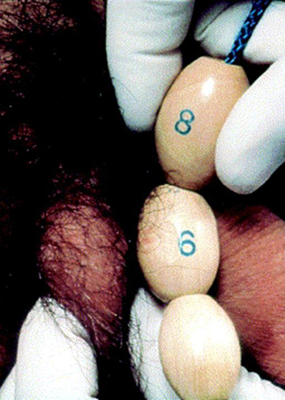
girls: breast development
The first demonstrable sign of puberty in females is breast development.[1]
pubic/axillary hair
Pubic and axillary hair, acne, and body odor develop as a result of androgens secreted from the adrenal gland, and partly from the ovary in females. The onset of axillary hair typically occurs in mid-puberty.
Pubic or axillary hair in the absence of breast development in girls or testicular enlargement in boys is typical in premature adrenarche, and on rare occasions can be a sign of congenital adrenal hyperplasia, adrenal gland tumors, or Cushing syndrome.
menarche
Menarche serves as a well-defined and documented evidence of puberty in girls. It typically occurs in stage 4 puberty in the majority of normal girls, within 2-3 years after the onset of breast development.
increased growth velocity
Estrogen, either from the ovary or aromatized from testicular testosterone, is the factor that mediates the increased growth hormone response during puberty.[41][42] The premature secretion of gonadal steroids in children results in the paradox of tall stature during childhood, due to an accelerated rate of linear growth, with normal or short stature as an adult, due to early fusion of the epiphyseal growth plates.
The growth velocity increases in mid to late puberty in boys and in early to mid puberty in girls.
On average, puberty contributes to 25 cm (10 inches) of height in females and 30 cm (12 inches) in males.
tall stature
The premature secretion of gonadal steroids in children results in the paradox of tall stature during childhood, due to an accelerated rate of linear growth, with normal or short stature as an adult, due to early fusion of the epiphyseal growth plates.
Other diagnostic factors
uncommon
café au lait spots
Skin examination should help identify café au lait spots present in neurofibromatosis type 1 (NF1), which have regular edges, and in McCune-Albright syndrome (MAS) that typically has irregular edges.
NF1 (with a high risk for optic gliomas) presents with central precocious puberty; MAS results in gonadotropin-independent precocious puberty. [Figure caption and citation for the preceding image starts]: Female with GIPP and café au lait hyperpigmented macules in McCune-Albright SyndromeFrom the collection of Dr A. Mehta; used with permission [Citation ends].
symptoms of other autonomous endocrine hyperfunction
Suggests McCune-Albright syndrome. Symptoms of other endocrine involvement include thyrotoxicosis (thyroid nodular hyperplasia), Cushing syndrome (multiple adrenal hyperplastic nodules), gigantism or acromegaly (pituitary adenoma and growth hormone excess), and galactorrhea (due to hyperprolactinemia).
polyostotic fibrous dysplasia
Component of McCune-Albright syndrome; a slowly progressive bone disorder that can involve any bone, with frequent facial asymmetry and hyperostosis of the base of the skull.
neurofibromas, axillary freckling, and kyphoscoliosis
Plexiform neurofibromas, axillary freckling, kyphoscoliosis, and a positive family history are indicative of neurofibromatosis type 1 causing central precocious puberty. [Figure caption and citation for the preceding image starts]: Axillary freckling in neurofibromatosis type IFrom the collection of Dr A. Mehta; used with permission [Citation ends].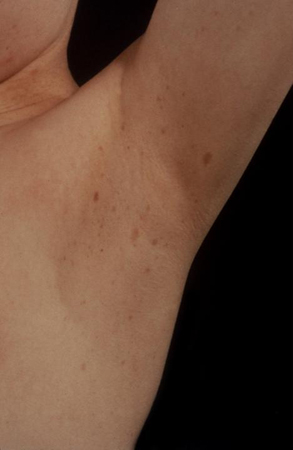
facial dysmorphism
Presence of dysmorphic features may reveal multisystem syndromes such as midline facial abnormalities in holoprosencephaly and septo-optic dysplasia. Facial asymmetry may be observed in McCune-Albright syndrome.
clitoromegaly
Suggests congenital adrenal hyperplasia or a virilizing adrenal or ovarian tumor as the underlying etiology causing gonadotropin-independent precocious puberty in females.
eye abnormalities
Include abnormalities such as Lisch nodules in neurofibromatosis type 1, or other visual deficits due to brain injury, tumors or infections, or optic nerve hypoplasia in septo-optic dysplasia.
motor deficits
A detailed neurologic examination should be performed. Focal motor deficits may be present due to intracranial tumors, previous head injury, hydrocephalus, meningitis, or encephalitis.
abnormal head size
Due to hydrocephalus, brain tumors, or central nervous system infections.
Risk factors
strong
brain tumors
Can provoke premature activation of the hypothalamo-pituitary-gonadal axis. These include tumors such as optic and hypothalamic gliomas, astrocytomas (these two are the most common), ependymomas, pineal tumors, and craniopharyngiomas.[27][28] Hamartomas of the tuber cinereum are congenital tumors composed of a heterotopic mass including gonadotropin-releasing hormone (GnRH) neurosecretory neurons; these are frequently associated with central precocious puberty and often occur before 3 years of age, particularly in males.
cranial irradiation
Moderate radiation doses (25-47.5 Gy) used for the treatment of brain tumors in children are associated with precocious puberty, with a direct relationship between age at pubertal onset and therapy.[29] Higher doses are, however, usually associated with gonadotropin deficiency.
McCune-Albright syndrome
A multisystem disorder characterized by the classic triad of gonadotropin-independent precocious puberty, hyperpigmented macules or café au lait spots, and polyostotic fibrous dysplasia. Although gonadal involvement occurs equally in girls and boys, the overproduction of sex steroids (and thus precocious puberty due to gonadotropin-independent precocious puberty) is much more common in females.
Two of these three features are required for the diagnosis. Autonomous hyperfunctioning most commonly involves the ovaries, but other endocrine involvement includes the thyroid (thyrotoxicosis), adrenals (Cushing syndrome), pituitary (gigantism/acromegaly or hyperprolactinemia), and parathyroid glands (hyperparathyroidism). The condition is sporadic and is caused by a somatic activating missense mutation in the gene encoding the alpha-subunit of the G-protein that stimulates cyclic adenosine monophosphate production.[Figure caption and citation for the preceding image starts]: Female with GIPP and café au lait hyperpigmented macules in McCune-Albright SyndromeFrom the collection of Dr A. Mehta; used with permission [Citation ends].
gonadal tumors
Can lead to gonadotropin-independent precocious puberty with high sex steroid concentrations. Types include granulosa cell tumors, Leydig cell tumors, gonadoblastomas, and adrenal virilizing tumors.
Human chorionic gonadotropin-secreting germ cell tumors are rare and cause precocious puberty in males only.[38] These tumors may occur in the gonads but can also occur in the brain (usually in the pineal region), liver, retroperitoneum, and posterior mediastinum.
Leydig cell tumors are associated with virilization and conversion of testosterone to estradiol leads to gynecomastia in males.
Granulosa cell and germ cell tumors can secrete both androgens and estradiol.
congenital adrenal hyperplasia (CAH)
Males with 21-hydroxylase CAH can present with gonadotropin-independent precocious puberty if undertreated. Females with 21-hydroxylase CAH present with signs of virilization (e.g., pubic and axillary hair, clitoromegaly) due to excess androgen but no breast development.
weak
positive family history
There is an association between the age at menarche in mothers and daughters.
In boys, familial testotoxicosis or male-limited gonadotropin-independent precocious puberty is associated with premature Leydig cell and germ cell maturation. It is inherited as an autosomal dominant condition that manifests only in males.[43][44]
Neurofibromatosis type 1(NF1) is an autosomal dominant condition with one affected parent.
exposure to exogenous hormones
Includes the oral contraceptive pill or testosterone gels. Estrogenic agents in cosmetics and food products have also been implicated in resulting in an earlier age of puberty; "epidemics" of premature thelarche (isolated breast development) in some geographic areas may be linked to an environmental exposure to estrogens.[22]
head injury
Can provoke premature activation of the hypothalamo-pituitary-gonadal axis and central precocious puberty.
previous central nervous system infections
Late complications of meningitis and encephalitis include early activation of the hypothalamo-pituitary-gonadal axis and central precocious puberty.
hydrocephalus
Can provoke premature activation of the hypothalamo-pituitary-gonadal axis and central precocious puberty.
congenital pituitary abnormalities
Caused by structural abnormalities (e.g., septo-optic dysplasia and holoprosencephaly). Early or precocious puberty may be isolated or combined with other pituitary hormone deficiencies.[34]
adoption
sexual abuse
Has been reported as a precipitating cause of central precocious puberty.[37] Early development can regress with a change in environment.
Use of this content is subject to our disclaimer