There are two aspects to the diagnosis: the recognition of an acute episode of MH, and the identification of patients with susceptibility to MH.
Recognition of an acute episode of MH
MH usually occurs during the intraoperative or, less commonly, in the postoperative period. Recognition of an acute episode is dependent on appropriate intra- and postoperative monitoring:
Minute ventilation, exhaled carbon dioxide (ETCO₂), and inspired and expired oxygen concentrations should be assessed. Minute ventilation (to maintain a normal end tidal carbon dioxide), carbon dioxide production, and oxygen consumption are all increased in an MH event. An unexplained and unexpected progressive increase in carbon dioxide production as evidenced in ETCO₂ should lead to a high index of suspicion for MH.[1]Hopkins PM, Girard T, Dalay S, et al. Malignant hyperthermia 2020: guideline from the Association of Anaesthetists. Anaesthesia. 2021 May;76(5):655-64.
https://www.doi.org/10.1111/anae.15317
http://www.ncbi.nlm.nih.gov/pubmed/33399225?tool=bestpractice.com
Core temperature should be monitored during all anesthetics. A marked increase in core temperature (>104°F [40°C]) can be seen in MH.[52]Larach MG, Brandom BW, Allen GC, et al. Malignant hyperthermia deaths related to inadequate temperature monitoring, 2007-2012: a report from the North American malignant hyperthermia registry of the malignant hyperthermia association of the United States. Anesth Analg. 2014 Dec;119(6):1359-66.
http://www.ncbi.nlm.nih.gov/pubmed/25268394?tool=bestpractice.com
[53]Shafer SL, Dexter F, Brull SJ. Deadly heat: economics of continuous temperature monitoring during general anesthesia. Anesth Analg. 2014 Dec;119(6):1235-7.
http://www.ncbi.nlm.nih.gov/pubmed/25405681?tool=bestpractice.com
Emphasis on temperature monitoring is stressed in every patient receiving an anesthetic, specifically when changes in body temperature are possible. An increase in deaths due to MH from 2007 to 2012 was reported to be likely associated with inadequate and inaccurate temperature monitoring.[53]Shafer SL, Dexter F, Brull SJ. Deadly heat: economics of continuous temperature monitoring during general anesthesia. Anesth Analg. 2014 Dec;119(6):1235-7.
http://www.ncbi.nlm.nih.gov/pubmed/25405681?tool=bestpractice.com
Unless the sympathetic nervous system response is obtunded, for example by the use of beta-blockers or remifentanil, increased carbon dioxide production accompanied by an otherwise unexplained and unexpected increase in heart rate is a diagnostic feature of MH. It is the upward trend in heart rate that is more useful than attainment of a specific value.[1]Hopkins PM, Girard T, Dalay S, et al. Malignant hyperthermia 2020: guideline from the Association of Anaesthetists. Anaesthesia. 2021 May;76(5):655-64.
https://www.doi.org/10.1111/anae.15317
http://www.ncbi.nlm.nih.gov/pubmed/33399225?tool=bestpractice.com
Muscle tone and the degree of neuromuscular blockade should be noted. Masseter muscle rigidity may be an early sign of an episode of MH.[2]Larach MG, Dirksen SJ, Belani KG, et al; Society for Ambulatory Anesthesiology; Malignant Hyperthermia Association of the United States; Ambulatory Surgery Foundation; Society for Academic Emergency Medicine; National Association of Emergency Medical Technicians. Special article: creation of a guide for the transfer of care of the malignant hyperthermia patient from ambulatory surgery centers to receiving hospital facilities. Anesth Analg. 2012 Jan;114(1):94-100.
http://journals.lww.com/anesthesia-analgesia/Fulltext/2012/01000/Creation_of_a_Guide_for_the_Transfer_of_Care_of.12.aspx
http://www.ncbi.nlm.nih.gov/pubmed/22052978?tool=bestpractice.com
[6]Larach MG, Gronert GA, Allen GC, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010 Feb 1;110(2):498-507.
http://journals.lww.com/anesthesia-analgesia/pages/articleviewer.aspx?year=2010&issue=02000&article=00039&type=Fulltext
http://www.ncbi.nlm.nih.gov/pubmed/20081135?tool=bestpractice.com
Patient’s body habitus and age should also be taken into consideration when suspecting acute MH. A review of pediatric patients found that there are differences in clinical characteristics of acute MH episodes among different-aged cohorts in childhood.[54]Nelson P, Litman RS. Malignant hyperthermia in children: an analysis of the North American malignant hyperthermia registry. Anesth Analg. 2014 Feb;118(2):369-74.
http://www.ncbi.nlm.nih.gov/pubmed/24299931?tool=bestpractice.com
Younger patients are likely to present with greater degree of lactic acidosis, while older children may demonstrate higher temperatures and potassium levels.
Any inhalation anesthetic should be stopped and fresh gas flow increased above minute ventilation if MH is suspected. Venous blood is obtained to measure venous blood gases and serum electrolytes, especially potassium. This should ideally be taken from a central venous catheter if feasible. Findings suggestive of MH include pCO₂ >55 mmHg (>7.33 kPa), pH <7.25, base excess more negative than -8 mEq/L, and potassium >6 mEq/L.[55]Larach MG, Localio AR, Allen GC, et al. A clinical grading scale to predict MH susceptibility. Anesthesiology. 1994 Apr;80(4):771-9.
http://www.ncbi.nlm.nih.gov/pubmed/8024130?tool=bestpractice.com
Hypercapnia, tachycardia, and muscle stiffness may abate after the inhalation anesthetic is removed, a therapeutic trial of dantrolene is given, and the patient is cooled to 100.4ºF (38ºC); this is supportive evidence that the patient has had an episode of MH. However, dantrolene can also decrease metabolism, temperature, heart rate, and acidosis in patients who are not susceptible to MH, so this response is not by itself diagnostic.
Rarely, MH may be triggered without exposure to anesthetics. Triggers include intense physical activity, exercise-induced rhabdomyolysis, febrile illness, or repeated episodes of heat-related illness.[8]Tobin JR, Jason DR, Challa VR, et al. Malignant hyperthermia and apparent heat stroke. JAMA. 2001 Jul 11;286(2):168-9.
http://www.ncbi.nlm.nih.gov/pubmed/11448278?tool=bestpractice.com
[10]Parness J. Hot on the trail of "I know it when I see it!". Anesth Analg. 2014 Feb;118(2):243-6.
http://www.ncbi.nlm.nih.gov/pubmed/24445622?tool=bestpractice.com
[11]Davis M, Brown R, Dickson A, et al. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br J Anaesth. 2002 Apr;88(4):508-15.
http://bja.oxfordjournals.org/content/88/4/508.full
http://www.ncbi.nlm.nih.gov/pubmed/12066726?tool=bestpractice.com
[13]Brown RL, Pollock AN, Couchman KG, et al. A novel ryanodine receptor mutation and genotype-phenotype correlation in a large malignant hyperthermia New Zealand Maori pedigree. Hum Mol Genet. 2000 Jun 12;9(10):1515-24.
http://hmg.oxfordjournals.org/content/9/10/1515.full
http://www.ncbi.nlm.nih.gov/pubmed/10888602?tool=bestpractice.com
Patients present with a spontaneous episode of severe muscle stiffness.[7]Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015 Aug 4;10:93.
http://ojrd.biomedcentral.com/articles/10.1186/s13023-015-0310-1
http://www.ncbi.nlm.nih.gov/pubmed/26238698?tool=bestpractice.com
MH should be considered in the differential diagnosis of any patient presenting with muscle stiffness associated with any of these triggers.[3]Sagui E, Montigon C, Abriat A, et al. Is there a link between exertional heat stroke and susceptibility to malignant hyperthermia? PLoS One. 2015 Aug 10;10(8):e0135496.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4530942
http://www.ncbi.nlm.nih.gov/pubmed/26258863?tool=bestpractice.com
[12]Groom L, Muldoon SM, Tang ZZ, et al. Identical de novo mutation in the type 1 ryanodine receptor gene associated with fatal, stress-induced malignant hyperthermia in two unrelated families. Anesthesiology. 2011 Nov;115(5):938-45.
http://www.ncbi.nlm.nih.gov/pubmed/21918424?tool=bestpractice.com
Confirmatory testing
Genetic testing or a muscle contracture test can provide a definitive diagnosis (see below). In settings where the clinical evidence very strongly supports a diagnosis of MH and a muscle contracture test is contraindicated (i.e., patient weighs <20 kg) genetic testing could precede muscle contracture testing.[56]Hopkins PM, Rüffert H, Snoeck MM, et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015 Oct;115(4):531-9.
https://www.emhg.org/testing-for-mh-1
http://www.ncbi.nlm.nih.gov/pubmed/26188342?tool=bestpractice.com
In these patients, a known MH-causative mutation may be found at loci RYR1, CACNA1S, or STAC3 (a skeletal muscle-specific T-tubule protein involved in calcium regulation). Further mutations are under investigation.
Exclusion of other diagnoses
Most of the signs of MH are nonspecific, so it is important to exclude other diagnoses. If the results of ventilation and routine blood tests are normal, the anesthetic complication is unlikely to be due to MH and other causes should be considered. The most important diagnoses to exclude depend on the abnormalities that are noted.
If hypercapnia is present, consider:
Changes in compliance of the ventilator system (leaks, faulty valves)
Changes in compliance of the patient's respiratory system (compromise of the upper airway, stiff lungs, elevated diaphragm, retraction against the lungs, mucus plugs)
Administration of drugs that increase the apneic threshold (narcotics, sedatives, anesthetics)
Delivery of carbon dioxide by insufflation
Increased metabolism due to other medical illnesses (sepsis, stimulant drugs, drug withdrawal, allergy, endocrinopathy).
If fever is present, consider:[57]Herlich A. Perioperative temperature elevation: not all hyperthermia is malignant hyperthermia. Paediatr Anaesth. 2013 Sep;23(9):842-50.
http://www.ncbi.nlm.nih.gov/pubmed/23890328?tool=bestpractice.com
Iatrogenic overheating
Inability to lose heat due to occlusive dressing, high humidity and temperature, or vasoconstriction
Diseases that increase the set point for thermoregulation
Presence of recently ingested illicit or designer drugs, also known as “new psychoactive substances”. These can alter neurotransmitter levels and result in hyperactivity leading to elevated temperatures.[58]Smith JP, Sutcliffe OB, Banks CE. An overview of recent developments in the analytical detection of new psychoactive substances (NPSs). Analyst. 2015 Aug 7;140(15):4932-48.
http://pubs.rsc.org/en/content/articlehtml/2015/an/c5an00797f
http://www.ncbi.nlm.nih.gov/pubmed/26031385?tool=bestpractice.com
If evidence of muscle injury is present (including high potassium), consider:
Conditions producing muscle ischemia
Possible history of inheritable muscular disease, such as dystrophinopathy
Concomitant myopathic drugs such as HMG-CoA reductase inhibitors (i.e., statins)
If there is evidence that rhabdomyolysis persists, this may be an indication of enzyme defects in the muscle (consider carnitine palmitoyltransferase [CPT] II deficiency and McArdle disease, muscle phosphorylase deficiency).
Monitoring for complications
Rhabdomyolysis
Urinalysis and urine myoglobin: should be performed as an initial test in all patients to detect rhabdomyolysis. If there is a positive finding on dipstick for blood, urine should be sent for microscopic and chemical analysis to differentiate between red blood cells, myoglobin, and hemoglobin.
Urine output should be monitored during the acute episode. A decrease in urine output accompanied by myoglobinemia indicates impending acute kidney injury.
Creatine kinase should be measured at the time of an episode of MH and daily until it is normal. If creatine kinase is markedly elevated and there have been minimal signs of increased metabolism, structural myopathies, such as dystrophinopathy, or enzyme defects, such as CPT deficiency, should be considered as causes.[36]Hirshey Dirksen SJ, Larach MG, Rosenberg H, et al. Future directions in malignant hyperthermia research and patient care. Anesth Analg. 2011 Nov;113(5):1108-19.
http://journals.lww.com/anesthesia-analgesia/Fulltext/2011/11000/Future_Directions_in_Malignant_Hyperthermia.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/21709147?tool=bestpractice.com
Acute kidney injury
Coagulopathy
Measures of coagulation function such as platelet count, prothrombin time, partial thromboplastin time, and fibrinogen should be performed as an initial test in all patients suspected of having an MH episode. MH susceptible people may be at increased risk of bleeding without exposure to pharmacologic MH triggers.[59]Lopez RJ, Byrne S, Vukcevic M, et al. An RYR1 mutation associated with malignant hyperthermia is also associated with bleeding abnormalities. Sci Signal. 2016 Jul 5;9(435):ra68.
http://www.ncbi.nlm.nih.gov/pubmed/27382027?tool=bestpractice.com
Consider thromboelastography if feasible.
Disseminated intravascular coagulation, leading to excessive bleeding, may be a complicating feature as MH progresses.[1]Hopkins PM, Girard T, Dalay S, et al. Malignant hyperthermia 2020: guideline from the Association of Anaesthetists. Anaesthesia. 2021 May;76(5):655-64.
https://www.doi.org/10.1111/anae.15317
http://www.ncbi.nlm.nih.gov/pubmed/33399225?tool=bestpractice.com
Identification of patients with susceptibility to MH
Susceptibility to MH is a subclinical condition, although patients may describe feeling uncomfortable exercising in the heat, and some patients may complain of muscle cramps.[12]Groom L, Muldoon SM, Tang ZZ, et al. Identical de novo mutation in the type 1 ryanodine receptor gene associated with fatal, stress-induced malignant hyperthermia in two unrelated families. Anesthesiology. 2011 Nov;115(5):938-45.
http://www.ncbi.nlm.nih.gov/pubmed/21918424?tool=bestpractice.com
Testing for susceptibility to MH by muscle contracture testing and/or genetic screening is part of basic health maintenance for families affected by MH. There can be discordance between genetic testing and muscle contracture testing results.[7]Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015 Aug 4;10:93.
http://ojrd.biomedcentral.com/articles/10.1186/s13023-015-0310-1
http://www.ncbi.nlm.nih.gov/pubmed/26238698?tool=bestpractice.com
[60]Robinson RL, Anetseder MJ, Brancadoro V, et al. Recent advances in the diagnosis of malignant hyperthermia susceptibility: how confident can we be of genetic testing? Eur J Human Genet. 2003 Apr;11(4):342-8.
http://www.nature.com/ejhg/journal/v11/n4/pdf/5200964a.pdf
http://www.ncbi.nlm.nih.gov/pubmed/12700608?tool=bestpractice.com
For diagnostic purposes the results of muscle contracture testing should be relied upon. However, when a causative genetic variant is identified, a diagnosis of malignant hyperthermia susceptibility should be applied and additional muscle contracture testing is not required.[56]Hopkins PM, Rüffert H, Snoeck MM, et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015 Oct;115(4):531-9.
https://www.emhg.org/testing-for-mh-1
http://www.ncbi.nlm.nih.gov/pubmed/26188342?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Diagnostic pathway for investigation of MH susceptibility. IVCT, in vitro contracture test; MH, malignant hyperthermia; MHN (MH negative or normal), classification applied when all contracture tests are negative; MHShc, MHSh, and MHSc, classifications are applied when contracture responses to both halothane and caffeine are abnormal, response to halothane alone is abnormal, or response to caffeine alone is abnormal, respectively. *Patients who should be asked to take part in research studies of the genetic basis of malignant hyperthermiaHopkins PM et al. Br J Anaesth. 2015 Oct;115(4):531-9. Used with permission [Citation ends].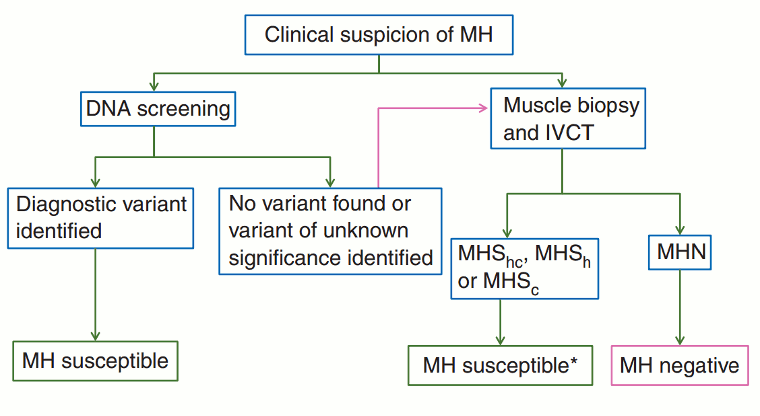
Muscle contracture testing
Should be carried out in all patients with a suspected recent episode of MH. This is an in vitro test that must be performed on fresh muscle, obtained by muscle biopsy. The patient must have recovered from the MH episode for this test to be valid. The patient must travel to the laboratory where the muscle test will be performed. The muscle biopsy and contracture test must be planned as any outpatient surgery. The in vitro administration of a bolus of halothane, or incremental exposure to caffeine, to the fresh muscle sample produces characteristic mechanical responses to stimulation in muscle susceptible to MH, which are diagnostic.[61]Metterlein T, Schuster F, Kranke P, et al. In-vitro contracture testing for susceptibility to malignant hyperthermia: can halothane be replaced? Eur J Anaesthesiol. 2011 Apr;28(4):251-5.
http://www.ncbi.nlm.nih.gov/pubmed/20827211?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: The mechanical response of normal and MH-susceptible muscle (positive) to direct stimulation in the presence of 3% halothaneFrom the collection of Dr Sheila Muldoon [Citation ends]. [Figure caption and citation for the preceding image starts]: The mechanical response of normal and MH-susceptible muscle (positive) to direct stimulation in the presence of incremental administration of caffeineFrom the collection of Dr Sheila Muldoon [Citation ends].
[Figure caption and citation for the preceding image starts]: The mechanical response of normal and MH-susceptible muscle (positive) to direct stimulation in the presence of incremental administration of caffeineFrom the collection of Dr Sheila Muldoon [Citation ends].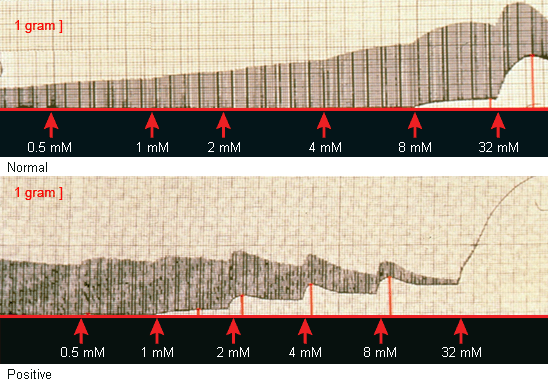
The caffeine halothane contracture test (CHCT) and the in vitro contracture test (IVCT) are the standardized bioassays used.[62]Islander G, Twetman ER. Comparison between the European and North American protocols for diagnosis of malignant hyperthermia susceptibility in humans. Anesth Analg. 1999 May;88(5):1155-60.
http://journals.lww.com/anesthesia-analgesia/Fulltext/1999/05000/Comparison_Between_the_European_and_North_American.35.aspx
http://www.ncbi.nlm.nih.gov/pubmed/10320187?tool=bestpractice.com
These tests are designed to be very sensitive and are the only tests that can definitively exclude the diagnosis of MH or verify the susceptibility to MH. The characteristic response is quantified as an increase in the contracture threshold. The diagnostic criteria differ depending on which test is used:
CHCT: patients with an increased contracture threshold in response to either caffeine or halothane are considered susceptible to MH.
IVCT: patients with an increased contracture threshold in response to caffeine and halothane, or either agent alone, are diagnosed as being susceptible to MH.[56]Hopkins PM, Rüffert H, Snoeck MM, et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015 Oct;115(4):531-9.
https://www.emhg.org/testing-for-mh-1
http://www.ncbi.nlm.nih.gov/pubmed/26188342?tool=bestpractice.com
The positive and negative predictive values of both of these contracture tests depend on the prior probability of the patient being susceptible to MH, which is established by a thorough review of the personal and family anesthetic and medical history.[7]Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015 Aug 4;10:93.
http://ojrd.biomedcentral.com/articles/10.1186/s13023-015-0310-1
http://www.ncbi.nlm.nih.gov/pubmed/26238698?tool=bestpractice.com
These tests require minor surgery and must be performed at an MH testing center, of which there are many worldwide.
Malignant Hyperthermia Association of the United States
Opens in new window
The European Malignant Hyperthermia Group
Opens in new window These tests have not been standardized for the pediatric population and because they require a large piece of muscle they are not suitable in children <20 kg.
Genetic testing
The role of genetic testing in MH is complex, yet rapidly expanding.[63]Stowell KM. DNA testing for malignant hyperthermia: the reality and the dream. Anesth Analg. 2014 Feb;118(2):397-406.
http://www.ncbi.nlm.nih.gov/pubmed/24445638?tool=bestpractice.com
[64]Riazi S, Kraeva N, Hopkins PM. Malignant hyperthermia in the post-genomics era: new perspectives on an old concept. Anesthesiology. 2018 Jan;128(1):168-80.
http://www.ncbi.nlm.nih.gov/pubmed/28902675?tool=bestpractice.com
Individuals who have been diagnosed susceptible to MH by muscle contracture testing should undergo genetic testing.
If there are existing genetic test results, do not order a duplicate test unless there is uncertainty about the existing result, e.g., the result is inconsistent with the patient’s clinical presentation or the test methodology has changed.[65]American College of Medical Genetics and Genomics. Five things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2021 [internet publication].
https://web.archive.org/web/20230326143738/https://www.choosingwisely.org/societies/american-college-of-medical-genetics-and-genomics
If MH is suspected as the cause of a death, genetic testing should be undertaken as part of the autopsy.[23]Gillies RL, Bjorksten AR, Du Sart D, et al. Analysis of the entire ryanodine receptor type 1 and alpha 1 subunit of the dihydropyridine receptor (CACNA1S) coding regions for variants associated with malignant hyperthermia in Australian families. Anaesth Intensive Care. 2015 Mar;43(2):157-66.
http://www.ncbi.nlm.nih.gov/pubmed/25735680?tool=bestpractice.com
[63]Stowell KM. DNA testing for malignant hyperthermia: the reality and the dream. Anesth Analg. 2014 Feb;118(2):397-406.
http://www.ncbi.nlm.nih.gov/pubmed/24445638?tool=bestpractice.com
Ryanodine receptor gene type 1 (RYR1) mutations have been found in MH-susceptible people in Europe, North America, and Japan. RYR1 mutations have also been found in families where MH and deaths occurred in South America, South Africa, Australia, New Zealand, Korea, and China. However, RYR1 mutations account for only 60% to 70% of MH cases.[66]Lanner JT. Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol. 2012;740:217-34.
http://www.ncbi.nlm.nih.gov/pubmed/22453944?tool=bestpractice.com
CACNA1S gene mutations may influence MH susceptibility in some families, although these account for less than 1% of MH cases.[7]Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015 Aug 4;10:93.
http://ojrd.biomedcentral.com/articles/10.1186/s13023-015-0310-1
http://www.ncbi.nlm.nih.gov/pubmed/26238698?tool=bestpractice.com
[23]Gillies RL, Bjorksten AR, Du Sart D, et al. Analysis of the entire ryanodine receptor type 1 and alpha 1 subunit of the dihydropyridine receptor (CACNA1S) coding regions for variants associated with malignant hyperthermia in Australian families. Anaesth Intensive Care. 2015 Mar;43(2):157-66.
http://www.ncbi.nlm.nih.gov/pubmed/25735680?tool=bestpractice.com
[67]Robinson RL, Curran JL, Ellis FR, et al. Multiple interacting gene products may influence susceptibility to malignant hyperthermia. Ann Hum Genet. 2000 Jul;64(Pt 4):307-20.
http://www.ncbi.nlm.nih.gov/pubmed/11415515?tool=bestpractice.com
Compound heterozygotes have also been identified.[17]Monnier N, Krivosic-Horber R, Payen JF, et al. Presence of two different genetic traits in malignant hyperthermia families: implications for genetics analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology. 2002 Nov;97(5):1067-74.
http://www.ncbi.nlm.nih.gov/pubmed/12411788?tool=bestpractice.com
[18]Ibarra MCA, Wu S, Murayama K, et al. Malignant hyperthermia in Japan: mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology. 2006 Jun;104(6):1146-54.
http://www.ncbi.nlm.nih.gov/pubmed/16732084?tool=bestpractice.com
[19]Brandom BW, Bina S, Wong CA, et al. Ryanodine receptor type 1 gene variants in the malignant hyperthermia-susceptible population of the United States. Anesth Analg. 2013 May;116(5):1078-86.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3633164
http://www.ncbi.nlm.nih.gov/pubmed/23558838?tool=bestpractice.com
Many families have novel mutations in RYR1.[11]Davis M, Brown R, Dickson A, et al. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br J Anaesth. 2002 Apr;88(4):508-15.
http://bja.oxfordjournals.org/content/88/4/508.full
http://www.ncbi.nlm.nih.gov/pubmed/12066726?tool=bestpractice.com
[13]Brown RL, Pollock AN, Couchman KG, et al. A novel ryanodine receptor mutation and genotype-phenotype correlation in a large malignant hyperthermia New Zealand Maori pedigree. Hum Mol Genet. 2000 Jun 12;9(10):1515-24.
http://hmg.oxfordjournals.org/content/9/10/1515.full
http://www.ncbi.nlm.nih.gov/pubmed/10888602?tool=bestpractice.com
[15]Chamley D, Pollock NA, Stowell KM, et al. Malignant hyperthermia in infancy and identification of novel RYR1 mutation. Br J Anaesth. 2000 Apr;84(4):500-4.
http://bja.oxfordjournals.org/content/84/4/500.full.pdf+html
http://www.ncbi.nlm.nih.gov/pubmed/10823104?tool=bestpractice.com
[18]Ibarra MCA, Wu S, Murayama K, et al. Malignant hyperthermia in Japan: mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology. 2006 Jun;104(6):1146-54.
http://www.ncbi.nlm.nih.gov/pubmed/16732084?tool=bestpractice.com
[19]Brandom BW, Bina S, Wong CA, et al. Ryanodine receptor type 1 gene variants in the malignant hyperthermia-susceptible population of the United States. Anesth Analg. 2013 May;116(5):1078-86.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3633164
http://www.ncbi.nlm.nih.gov/pubmed/23558838?tool=bestpractice.com
[24]Sambuughin N, Holley H, Muldoon S, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology. 2005 Mar;102(3):515-21.
http://www.ncbi.nlm.nih.gov/pubmed/15731587?tool=bestpractice.com
[25]Levano S, Vukcevic M, Singer M, et al. Increasing the number of diagnostic mutations in malignant hyperthermia. Human Mutat. 2009 Apr;30(4):590-8.
http://www.ncbi.nlm.nih.gov/pubmed/19191329?tool=bestpractice.com
[68]Schiemann AH, Paul N, Parker R, et al. Functional characterization of 2 known ryanodine receptor mutations causing malignant hyperthermia. Anesth Analg. 2014 Feb;118(2):375-80.
http://www.ncbi.nlm.nih.gov/pubmed/24361844?tool=bestpractice.com
These are suspected to cause MH but are not biologically confirmed.[33]Brislin RP, Theroux MC. Core myopathies and malignant hyperthermia susceptibility: a review. Paediatr Anaesth. 2013 Sep;23(9):834-41.
http://www.ncbi.nlm.nih.gov/pubmed/23617272?tool=bestpractice.com
[69]Kaufmann A, Kraft B, Michalek-Sauberer A, et al. Novel double and single ryanodine receptor 1 variants in two Austrian malignant hyperthermia families. Anesth Analg. 2012 May;114(5):1017-25.
http://www.ncbi.nlm.nih.gov/pubmed/22415532?tool=bestpractice.com
Given the variable penetrance and prevalence of malignant hyperthermia-susceptible (MHS) individuals, new genetic techniques such as exome sequencing have identified unsuspecting families as MHS.[20]Gonsalves SG, Ng D, Johnston JJ, et al; NISC Comparative Sequencing Program. Using exome data to identify malignant hyperthermia susceptibility mutations. Anesthesiology. 2013 Nov;119(5):1043-53.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4077354
http://www.ncbi.nlm.nih.gov/pubmed/24195946?tool=bestpractice.com
[32]Klingler W, Heiderich S, Girard T, et al. Functional and genetic characterization of clinical malignant hyperthermia crises: a multi-centre study. Orphanet J Rare Dis. 2014 Jan 16;9:8.
http://www.ojrd.com/content/9/1/8
http://www.ncbi.nlm.nih.gov/pubmed/24433488?tool=bestpractice.com
[63]Stowell KM. DNA testing for malignant hyperthermia: the reality and the dream. Anesth Analg. 2014 Feb;118(2):397-406.
http://www.ncbi.nlm.nih.gov/pubmed/24445638?tool=bestpractice.com
Genetic counseling is indicated in all patients with mutations suspected to cause susceptibility to MH, including those of unknown significance.[10]Parness J. Hot on the trail of "I know it when I see it!". Anesth Analg. 2014 Feb;118(2):243-6.
http://www.ncbi.nlm.nih.gov/pubmed/24445622?tool=bestpractice.com
Genetic testing is carried out at a specialized center.[56]Hopkins PM, Rüffert H, Snoeck MM, et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015 Oct;115(4):531-9.
https://www.emhg.org/testing-for-mh-1
http://www.ncbi.nlm.nih.gov/pubmed/26188342?tool=bestpractice.com
Malignant Hyperthermia Association of the United States
Opens in new window
The European Malignant Hyperthermia Group
Opens in new window
Screening of relatives
If a patient is diagnosed as susceptible to MH, close blood relatives should be screened once the assessment of the patient is complete.[23]Gillies RL, Bjorksten AR, Du Sart D, et al. Analysis of the entire ryanodine receptor type 1 and alpha 1 subunit of the dihydropyridine receptor (CACNA1S) coding regions for variants associated with malignant hyperthermia in Australian families. Anaesth Intensive Care. 2015 Mar;43(2):157-66.
http://www.ncbi.nlm.nih.gov/pubmed/25735680?tool=bestpractice.com
If the patient has an identifiable mutation, genetic studies on relatives can be focused on the particular exon.
If the same mutation is identified in a relative, that individual should be considered to be susceptible to MH, and muscle contracture testing is not required. If a mutation is not identified, then muscle contracture testing is required to definitively exclude the diagnosis.[70]Girard T, Treves S, Voronkov E, et al. Molecular genetic testing for malignant hyperthermia susceptibility. Anesthesiology. 2004 May;100(5):1076-80.
http://www.ncbi.nlm.nih.gov/pubmed/15114203?tool=bestpractice.com
Detection of muscle enzyme deficiencies
MH is commonly associated with an increase in creatine kinase, and some patients with susceptibility to MH will have chronic elevations. If a patient has a recurrent elevation in creatine kinase more than 5 times the upper limit of normal, muscle enzyme deficiencies associated with rhabdomyolysis should be investigated.[36]Hirshey Dirksen SJ, Larach MG, Rosenberg H, et al. Future directions in malignant hyperthermia research and patient care. Anesth Analg. 2011 Nov;113(5):1108-19.
http://journals.lww.com/anesthesia-analgesia/Fulltext/2011/11000/Future_Directions_in_Malignant_Hyperthermia.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/21709147?tool=bestpractice.com
[49]Landau ME, Kenney K, Deuster P, et al. Exertional rhabdomyolysis: a clinical review with a focus on genetic influences. J Clin Neuromuscul Dis. 2012 Mar;13(3):122-36.
http://www.ncbi.nlm.nih.gov/pubmed/22538307?tool=bestpractice.com
The exercise intolerance panel includes specific tests for these deficiencies, including carnitine palmitoyltransferase 2 (CPT2) deficiency, myophosphorylase deficiency, and myoadenylate deaminase deficiency.

 [Figure caption and citation for the preceding image starts]: The mechanical response of normal and MH-susceptible muscle (positive) to direct stimulation in the presence of incremental administration of caffeineFrom the collection of Dr Sheila Muldoon [Citation ends].
[Figure caption and citation for the preceding image starts]: The mechanical response of normal and MH-susceptible muscle (positive) to direct stimulation in the presence of incremental administration of caffeineFrom the collection of Dr Sheila Muldoon [Citation ends].