Tests
1st tests to order
nerve conduction studies
Test
Initial compound muscle action potential amplitude is typically low in Lambert-Eaton myasthenic syndrome (LEMS). After 10 seconds of exercise, facilitation of ≥100% increase in amplitude is found in at least one muscle in 90% of patients with LEMS; facilitation varies but is greater in distal muscles.[26][36][Figure caption and citation for the preceding image starts]: Compound muscle action potentials (abductor digiti quinti manus muscle) following ulnar nerve stimulation: (A) at rest, (B) immediately after 10 seconds of maximum voluntary contraction demonstrating 1500% postexercise facilitationFrom the collection of Dr Vern C. Juel [Citation ends].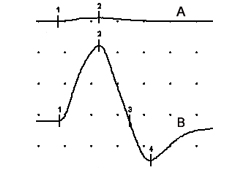
Result
doubling of compound muscle action potential amplitude postexercise
low-frequency repetitive nerve stimulation
Test
Low-frequency repetitive nerve stimulation approaches 100% sensitivity in distal limb muscles.[22][23] False-negatives may be seen in cool or incompletely relaxed limbs. Relatively less specific because test may be positive for other neuromuscular junction disorders (e.g., myasthenia gravis) and motor neuropathic processes (e.g., amyotrophic lateral sclerosis).
Result
>10% compound muscle action potential amplitude decrement between the first and fourth compound muscle action potential; the decrement is progressive and typically does not have the classic “saddle-shape” seen in myasthenia gravis
anti-P/Q voltage-gated calcium-channel serology
chest CT scan
Test
Should be ordered for all patients with suspected cancer-associated Lambert-Eaton myasthenic syndrome.[34] Cancer is found either at disease onset or subsequently in around 50% of patients.[2][8][9]
Small cell lung cancer is the most commonly associated cancer, although an association with lymphoproliferative disorders has also been described.[3]
Result
variable; may show evidence of underlying malignancy
anti-acetylcholine receptor (AChR) serology
Test
The presence of AChR or MuSK antibodies strongly suggests myasthenia gravis, although up to 13% of patients with Lambert-Eaton myasthenic syndrome (LEMS) have AChR antibodies, and myasthenia gravis/LEMS overlap syndromes do occur rarely.[33]
Result
variable; usually negative
thyroid-stimulating hormone (TSH)
Test
Performed in all patients with Lambert-Eaton myasthenic syndrome to assess for comorbid thyroid dysfunction.
Result
may be abnormal
Tests to consider
serial PFTs
Test
Serial measurements of FVC and negative inspiratory force should be taken for patients with severe bulbar weakness.
Indication for mechanical ventilation includes FVC of ≤15 mL/kg and negative inspiratory force of ≤20 cm H₂O.
Neither abnormal ABGs nor pulse oxygenation reflect the degree of respiratory weakness because abnormalities in either occur late in the course after clinical decompensation.
Result
low FVC and negative inspiratory force in patients with respiratory crisis
total-body fluoro-2-deoxyglucose positron emission tomography (FDG-PET) scan
Test
Lambert-Eaton myasthenic syndrome (LEMS) precedes the diagnosis of small cell lung cancer (SCLC) in up to 69% of patients with LEMS; SCLC is subsequently diagnosed in up to 96% of patients within 1 year of diagnosis.[7][8] Total-body FDG-PET may detect SCLC when chest CT is normal.[8][34][35]
Result
variable; may show evidence of underlying malignancy
bronchoscopy
high-frequency or tetanic repetitive nerve stimulation (RNS)
Test
RNS performed at 20-50 Hz to demonstrate tetanic facilitation has no significant diagnostic superiority to eliciting postexercise facilitation with 10 seconds of maximum voluntary isometric exercise; ≥100% postexercise or tetanic facilitation with high-frequency RNS in the abductor digiti quinti manus muscle is fairly specific for the diagnosis of Lambert-Eaton myasthenic syndrome (LEMS).[23][26] In LEMS, the postexercise facilitation lasts a few seconds, as opposed to botulism where facilitation may be sustained for several minutes.
Result
doubling of amplitude postexercise
single-fiber electromyography
Test
Highly sensitive investigation for neuromuscular junction dysfunction.[26][27]
As in other presynaptic neuromuscular junction disorders (e.g., botulism), jitter and blocking in Lambert-Eaton myasthenic syndrome are rate-dependent and improve with higher rates of motor axonal firing, either with voluntary activation or with axonal stimulation.
Result
increased variability in time intervals between muscle fiber action potentials (jitter) or complete failure of neuromuscular transmission (blocking)
HLA haplotyping
Test
Associated HLA haplotypes may be assessed. Presence of specific haplotypes is not diagnostic but may be of some value in distinguishing non-cancer-associated Lambert-Eaton myasthenic syndrome (NCA-LEMS) from cancer-associated LEMS (CA-LEMS).
In NCA-LEMS, a higher frequency of HLA-DR3, -B8, or -A1 is seen (HLA-DR3, 67%; -B8, 64%; -A1, 52%) than in patients with CA-LEMS (HLA-DR, 30%; -B8, 20%; -A1, 18%).[7] Forty-one percent of patients with NCA-LEMS have all three haplotypes compared with 5% of patients with CA-LEMS.[7]
Result
variable; may show HLA-DR3, -B8, or -A1 haplotype
antinuclear antibodies (ANA)
Test
Performed in patients with clinical features atypical for Lambert-Eaton myasthenic syndrome suggestive of a superimposed autoimmune disorder (e.g., systemic lupus erythematosus).
Result
may be positive
rheumatoid factor (RF)
Test
Performed in patients with clinical features atypical for Lambert-Eaton myasthenic syndrome suggestive of a superimposed autoimmune disorder (e.g., rheumatoid arthritis).
Result
may be positive
antineutrophil cytoplasmic autoantibodies (ANCA)
Test
Performed in patients with clinical features atypical for Lambert-Eaton myasthenic syndrome suggestive of a superimposed autoimmune disorder (e.g., systemic vasculitis).
Result
may be positive
B12 and methylmalonic acid (MMA)
Test
Performed in patients with clinical features or macrocytic anemia suggestive of B12 deficiency in the context of Lambert-Eaton myasthenic syndrome.
Result
B12 may be low; MMA may be elevated
Emerging tests
alpha-1A P/Q voltage-gated calcium-channel subunit antibodies
Test
Antibody response is highly suggestive of non-cancer-associated Lambert-Eaton myasthenic syndrome (NCA-LEMS) (38% of patients with NCA-LEMS compared with 5% of patients with cancer-associated LEMS).[31]
Result
variable; may be positive
anti-SOX1 antibodies
Test
Sixty-four percent sensitivity in cancer-associated Lambert-Eaton myasthenic syndrome (CA-LEMS), although also seen in Hu-positive paraneoplastic neurologic syndromes (32%) and small cell lung cancer (SCLC) without neurologic symptoms (22%).[32] Rarely seen in non-cancer-associated LEMS; detection of the antibody is highly suggestive of SCLC.
Result
variable; may be positive
Use of this content is subject to our disclaimer