Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
Look out for this icon: for treatment options that are affected, or added, as a result of your patient's comorbidities.
all patients
1st line – assessment of airway, breathing, and circulation
assessment of airway, breathing, and circulation
Assessment and treatment proceed alongside each other. At this stage the etiology of shock may not be clear. Key first steps are to ensure a patent airway (through intubation if required) and to optimize oxygen delivery with supplemental oxygen, commonly aiming for arterial oxygen saturations of 94% to 98%.[34]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com Routine supplementation of oxygen to achieve higher oxygen saturations has been linked to coronary and pulmonary arterial vasoconstriction in acute myocardial infarction.[68]McNulty PH, King N, Scott S, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1057-62. https://journals.physiology.org/doi/full/10.1152/ajpheart.00625.2004 http://www.ncbi.nlm.nih.gov/pubmed/15706043?tool=bestpractice.com [69]Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976 May 8;1(6018):1121-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1639993 http://www.ncbi.nlm.nih.gov/pubmed/773507?tool=bestpractice.com For patients with sepsis-induced hypoxemic respiratory failure, high-flow nasal oxygen is recommended over noninvasive ventilation.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com
Continuous monitoring is imperative to monitor response to therapy and to guide treatment. This includes clinical observation, repeated blood pressure readings, respiratory rate, oxygen saturations, pulse, level of consciousness, and monitoring of cardiac rhythm. In the critical care setting, continuous capnography can aid the assessment of shock.[36]Nassar BS, Schmidt GA. Capnography during critical illness. Chest. 2016 Feb;149(2):576-85. http://www.ncbi.nlm.nih.gov/pubmed/26447854?tool=bestpractice.com Side stream or direct capnography (when endotracheal airway is in place) can be helpful in assessing pulmonary perfusion matched to ventilation.[36]Nassar BS, Schmidt GA. Capnography during critical illness. Chest. 2016 Feb;149(2):576-85. http://www.ncbi.nlm.nih.gov/pubmed/26447854?tool=bestpractice.com The lactate level will usually decrease if the patient is clinically improving.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com Treatment is dependent on continuous monitoring of these variables and guided by their response.
treatment of underlying cause
Treatment recommended for ALL patients in selected patient group
The underlying cause of shock needs to be identified as rapidly as possible. It indicates the most critical treatment priorities.
Identification of cardiogenic shock is important because aggressive fluid administration may worsen the shock state and lead to the onset (or worsening) of acute pulmonary edema. Early involvement of specialist multidisciplinary teams (e.g., heart failure and critical care specialists, interventional cardiologists, and cardiac surgeons) is recommended when cardiogenic shock is suspected.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com
In cardiogenic shock secondary to a large myocardial infarction, urgent revascularization of the coronary arteries, by percutaneous coronary intervention, direct coronary infusion of thrombolytics, or cardiovascular surgery, lowers mortality.[50]van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017 Oct 17;136(16):e232-68. http://circ.ahajournals.org/content/136/16/e232.long http://www.ncbi.nlm.nih.gov/pubmed/28923988?tool=bestpractice.com [84]Hochman JS, Sleeper LA, Webb JG, et al; SHOCK Investigators. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006 Jun 7;295(21):2511-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1782030 http://www.ncbi.nlm.nih.gov/pubmed/16757723?tool=bestpractice.com
Severe rhythm disturbances should be corrected urgently in patients with acute heart failure and unstable conditions, using medical therapy, electrical cardioversion, or temporary pacing.[3]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726. https://academic.oup.com/eurheartj/article/42/36/3599/6358045 http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
Cardiac tamponade needs drainage; pericardiocentesis under ECG monitoring can have an effect by draining as little as 30 mL, but this may be unsuccessful if the blood is clotted. A pericardial drain or surgical pericardial window may be required.
In pulmonary embolism, shock is the most widely accepted indication for thrombolysis.[110]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90. https://www.sciencedirect.com/science/article/pii/S0735109716000115?via%3Dihub http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com [111]Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016 Feb;149(2):315-52. https://journal.chestnet.org/article/S0012-3692(15)00335-9/fulltext http://www.ncbi.nlm.nih.gov/pubmed/26867832?tool=bestpractice.com Emergency embolectomy is an alternative, especially when thrombolysis is contraindicated or has failed.
For anaphylactic shock, intramuscular epinephrine (adrenaline) is recommended as the most important treatment by all major guidelines.[63]Golden DBK, Wang J, Waserman S, et al. Anaphylaxis: a 2023 practice parameter update. Ann Allergy Asthma Immunol. 2024 Feb;132(2):124-76. https://www.annallergy.org/article/S1081-1206(23)01304-2/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38108678?tool=bestpractice.com [64]Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020 Oct 20;142(16_suppl_2):S366-S468. https://www.doi.org/10.1161/CIR.0000000000000916 http://www.ncbi.nlm.nih.gov/pubmed/33081529?tool=bestpractice.com [65]Lott C, Truhlář A, Alfonzo A, et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation. 2021 Apr;161:152-219. https://www.doi.org/10.1016/j.resuscitation.2021.02.011 http://www.ncbi.nlm.nih.gov/pubmed/33773826?tool=bestpractice.com
Early recognition and treatment of septic shock is key to improving outcomes. Current best practice is based on evidence for care bundles in sepsis.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com [89]Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med. 2015 Sep;41(9):1620-8. http://www.ncbi.nlm.nih.gov/pubmed/26109396?tool=bestpractice.com [90]Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014 Nov;40(11):1623-33. https://link.springer.com/article/10.1007%2Fs00134-014-3496-0 http://www.ncbi.nlm.nih.gov/pubmed/25270221?tool=bestpractice.com [91]Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017 Jun 8;376(23):2235-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5538258 http://www.ncbi.nlm.nih.gov/pubmed/28528569?tool=bestpractice.com The Surviving Sepsis Campaign (SSC) treatment guidelines remain the most widely accepted standards.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com The SSC guidelines recommend obtaining blood cultures prior to the administration of antibiotics; administering antimicrobials immediately, ideally within 1 hour of recognition (in adults with possible septic shock or a high likelihood for sepsis); administering empiric antimicrobials with MRSA coverage in patients with sepsis or septic shock at high risk of MRSA; administering 30 mL/kg crystalloid for hypotension within the first 3 hours of resuscitation; obtaining serial measurement of blood lactate; aiming for a mean arterial pressure of ≥65 mmHg in patients with septic shock on vasopressors. For adults with an initial diagnosis of sepsis or septic shock, and adequate source control where optimal duration of therapy is unclear, a combination of procalcitonin and clinical evaluation is recommended to decide when to discontinue antimicrobials.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com The SSC suggests intravenous corticosteroids in adults with septic shock and an ongoing requirement for vasopressor therapy.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com
Hemorrhagic shock due to trauma requires a team approach with attention to identification of cause for, and control of, hemorrhage as soon as possible. Massive hemorrhage due to trauma is often associated with fibrinolysis, which further exacerbates hemorrhage by inhibiting clot formation. Antifibrinolytic agents may have a beneficial effect in addition to fluid and blood transfusion in stabilizing a trauma patient with shock.[92]Ker K, Roberts I, Shakur H, et al. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015 May 9;(5):CD004896. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004896.pub4/full http://www.ncbi.nlm.nih.gov/pubmed/25956410?tool=bestpractice.com Overly aggressive fluid infusion may increase the bleeding rate in hemorrhagic shock: in particular, when mean arterial pressures are >40 mmHg.[79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90. http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com
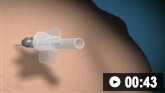
Tension pneumothorax requires urgent decompression through needle thoracocentesis.
How to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous catheter into the fourth intercostal space in an adult.
vasoactive agents
Treatment recommended for SOME patients in selected patient group
Short-term intravenous infusion of a vasoactive agent (vasopressor and/or inotrope) should be considered in patients with hypotension (systolic BP <90mmHg) and/or signs or symptoms of hypoperfusion, despite adequate filling status.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com
Patients requiring vasoactive drugs (vasopressors/inotropes) need continuous monitoring in a critical care setting. Vasoactive agents may cause tachycardia, and induce arrhythmias and myocardial ischemia.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com Selection of appropriate vasoactive agents should only take place under critical care supervision, and may vary according to the type of shock, clinician preference, and local practice guidelines.
Intravenous inotropic support can increase cardiac output and improve hemodynamics in patients presenting with cardiogenic shock.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com There is a lack of robust evidence to suggest the clear benefit of one inotropic agent over another in cardiogenic shock.[80]Kivikko M, Pollesello P, Tarvasmäki T, et al. Effect of baseline characteristics on mortality in the SURVIVE trial on the effect of levosimendan vs dobutamine in acute heart failure: Sub-analysis of the Finnish patients. Int J Cardiol. 2016 Jul 15;215:26-31. https://www.doi.org/10.1016/j.ijcard.2016.04.064 http://www.ncbi.nlm.nih.gov/pubmed/27107540?tool=bestpractice.com In general, the choice of a specific inotropic agent is guided by blood pressure, concurrent arrhythmias, and availability of drug.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com Inotropes should be used with caution because there is evidence that they result in increased mortality.[3]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726. https://academic.oup.com/eurheartj/article/42/36/3599/6358045 http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com [4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com [81]Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul 5;46(1):57-64. https://www.doi.org/10.1016/j.jacc.2005.03.051 http://www.ncbi.nlm.nih.gov/pubmed/15992636?tool=bestpractice.com Inotropes should be discontinued if there are sustained arrhythmias or symptomatic coronary ischemia. Continuous monitoring of cardiac rhythm is recommended during infusion of inotropes.
Vasopressors are commonly recommended only after adequate volume resuscitation.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com [3]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726. https://academic.oup.com/eurheartj/article/42/36/3599/6358045 http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com Dose is commonly titrated to achieve a mean arterial blood pressure of ≥65 mmHg, or a systolic blood pressure of ≥90 mmHg.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com [79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90. http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com [82]Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014 Apr 24;370(17):1583-93. http://www.ncbi.nlm.nih.gov/pubmed/24635770?tool=bestpractice.com Vasopressors increase the risk of tissue ischemia and necrosis in a dose-dependent manner.
Consult a specialist for guidance on suitable vasopressor/inotrope regimens and doses.
intravenous fluids
Treatment recommended for ALL patients in selected patient group
Evidence-based guidelines for the management of septic shock recommend 30mL/kg intravenous crystalloid fluid within the first 3 hours.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com Ongoing management is guided by the continuous monitoring of organ perfusion and hemodynamics. Resuscitation in sepsis should be guided by dynamic measures rather than by physical exam or static measures alone. Dynamic parameters include the response to a passive leg raise or a fluid bolus, using stroke volume, stroke volume variation, pulse pressure variation, or echocardiography, where available. Predicting the effect of further volume in any individual patient requires considerable clinical judgment because no single, simple, and precise measure has been yet identified. Central venous pressure and ultrasonic parameters (central inferior vena cava diameter and collapsibility and aortic blood flow) are useful.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643 http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com [66]Nieminen MS, Böhm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. Eur Heart J. 2005 Feb;26(4):384-416. http://eurheartj.oxfordjournals.org/cgi/content/full/26/4/384 http://www.ncbi.nlm.nih.gov/pubmed/15681577?tool=bestpractice.com [79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90. http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com Commonly, systolic blood pressure of 90 mmHg or a mean arterial pressure of 65 mmHg is targeted during fluid volume resuscitation; infusion is stopped if concerns of volume overload develop (pulmonary rales or suspected pulmonary edema).
The major risk with volume resuscitation is pulmonary edema. In hemorrhagic shock, overly aggressive fluid infusion may increase the bleeding rate, in particular when mean arterial pressures are >40 mmHg.[79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90. http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com
If there is no evidence for, or suspicion of, pulmonary edema secondary to cardiac failure, early fluid delivery is more important than the type of fluid (crystalloid or colloid).[71]Finfer S, Bellomo R, Boyce N, et al; the SAFE study investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004 May 27;350(22):2247-56.
http://www.nejm.org/doi/full/10.1056/NEJMoa040232#t=article
http://www.ncbi.nlm.nih.gov/pubmed/15163774?tool=bestpractice.com
[72]Roberts I, Blackhall K, Alderson P, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2011 Nov 9;(11):CD001208.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001208.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/22071799?tool=bestpractice.com
[73]Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018 Aug 3;(8):CD000567.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub7/full
http://www.ncbi.nlm.nih.gov/pubmed/30073665?tool=bestpractice.com
There is no evidence for any survival benefit of colloid fluids over crystalloid.[73]Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018 Aug 3;(8):CD000567.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub7/full
http://www.ncbi.nlm.nih.gov/pubmed/30073665?tool=bestpractice.com
[112]Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998 Mar 28;316(7136):961-4.
http://www.bmj.com/cgi/content/full/316/7136/961
http://www.ncbi.nlm.nih.gov/pubmed/9550953?tool=bestpractice.com
[113]Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999 Jan;27(1):200-10.
http://www.ncbi.nlm.nih.gov/pubmed/9934917?tool=bestpractice.com
[114]Akech S, Ledermann H, Maitland K. Choice of fluids for resuscitation in children with severe infection and shock: systematic review. BMJ. 2010 Sep 2;341:c4416.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2933356
http://www.ncbi.nlm.nih.gov/pubmed/20813823?tool=bestpractice.com
[115]Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014 Apr 10;370(15):1412-21.
http://www.nejm.org/doi/full/10.1056/NEJMoa1305727#t=article
http://www.ncbi.nlm.nih.gov/pubmed/24635772?tool=bestpractice.com
[  ]
How do colloids compare with crystalloids for fluid resuscitation in critically ill people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2307/fullShow me the answer[Evidence B]dcee690f-801f-4e69-9558-b7db51437fe3ccaBHow do colloids compare with crystalloids for fluid resuscitation in critically ill people? There is some evidence that balanced crystalloids may be preferable to normal saline in critically ill patients in intensive care, although other studies have found no difference.[76]Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
https://www.nejm.org/doi/full/10.1056/NEJMoa1711584?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://www.ncbi.nlm.nih.gov/pubmed/29485925?tool=bestpractice.com
[116]Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021 Aug 10;326(9):1-12.
https://jamanetwork.com/journals/jama/fullarticle/2783039
http://www.ncbi.nlm.nih.gov/pubmed/34375394?tool=bestpractice.com
The Surviving Sepsis Campaign recommends balanced crystalloids in patients with sepsis or septic shock.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643
http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com
In July 2021, the US Food and Drug Administration (FDA) issued safety labeling changes for solutions containing hydroxyethyl starch (HES) stating that HES products should not be used unless adequate alternative treatment is unavailable.[74]US Food and Drug Administration. Labeling changes on mortality, kidney injury, and excess bleeding with hydroxyethyl starch products. Jul 2021 [internet publication].
https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products
HES products are associated with adverse outcomes including kidney injury and death, particularly in critically ill patients and those with sepsis.[79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90.
http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com
[117]Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013 Feb 20;309(7):678-88.
https://jamanetwork.com/journals/jama/fullarticle/1653505
http://www.ncbi.nlm.nih.gov/pubmed/23423413?tool=bestpractice.com
In view of the serious risks posed to these patient populations, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency in February 2022 recommended suspending HES solutions for infusion in Europe.[75]European Medicines Agency. PRAC recommends suspending hydroxyethyl-starch solutions for infusion from the market. Feb 2022 [internet publication].
https://www.ema.europa.eu/en/news/prac-recommends-suspending-hydroxyethyl-starch-solutions-infusion-market-0
The Surviving Sepsis Campaign advises against the use of starches for patients with sepsis and septic shock.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643
http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com
]
How do colloids compare with crystalloids for fluid resuscitation in critically ill people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2307/fullShow me the answer[Evidence B]dcee690f-801f-4e69-9558-b7db51437fe3ccaBHow do colloids compare with crystalloids for fluid resuscitation in critically ill people? There is some evidence that balanced crystalloids may be preferable to normal saline in critically ill patients in intensive care, although other studies have found no difference.[76]Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
https://www.nejm.org/doi/full/10.1056/NEJMoa1711584?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://www.ncbi.nlm.nih.gov/pubmed/29485925?tool=bestpractice.com
[116]Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021 Aug 10;326(9):1-12.
https://jamanetwork.com/journals/jama/fullarticle/2783039
http://www.ncbi.nlm.nih.gov/pubmed/34375394?tool=bestpractice.com
The Surviving Sepsis Campaign recommends balanced crystalloids in patients with sepsis or septic shock.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643
http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com
In July 2021, the US Food and Drug Administration (FDA) issued safety labeling changes for solutions containing hydroxyethyl starch (HES) stating that HES products should not be used unless adequate alternative treatment is unavailable.[74]US Food and Drug Administration. Labeling changes on mortality, kidney injury, and excess bleeding with hydroxyethyl starch products. Jul 2021 [internet publication].
https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products
HES products are associated with adverse outcomes including kidney injury and death, particularly in critically ill patients and those with sepsis.[79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90.
http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com
[117]Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013 Feb 20;309(7):678-88.
https://jamanetwork.com/journals/jama/fullarticle/1653505
http://www.ncbi.nlm.nih.gov/pubmed/23423413?tool=bestpractice.com
In view of the serious risks posed to these patient populations, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency in February 2022 recommended suspending HES solutions for infusion in Europe.[75]European Medicines Agency. PRAC recommends suspending hydroxyethyl-starch solutions for infusion from the market. Feb 2022 [internet publication].
https://www.ema.europa.eu/en/news/prac-recommends-suspending-hydroxyethyl-starch-solutions-infusion-market-0
The Surviving Sepsis Campaign advises against the use of starches for patients with sepsis and septic shock.[2]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-247.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8486643
http://www.ncbi.nlm.nih.gov/pubmed/34599691?tool=bestpractice.com
blood products
Treatment recommended for SOME patients in selected patient group
Indicated in patients with acute hemorrhage or profound anemia from chronic red blood cell loss.[78]Association of Anaesthetists. AAGBI guidelines: the use of blood components and their alternatives 2016. April 2016 [internet publication]. https://associationofanaesthetists-publications.onlinelibrary.wiley.com/doi/10.1111/anae.13489
tranexamic acid
Treatment recommended for SOME patients in selected patient group
For patients with severe hemorrhage caused by trauma, tranexamic acid can be used within 3 hours of the initial injury. A meta-analysis found strong evidence that treatment should be immediate for maximum survival benefit, with even a short delay associated with higher mortality.[118]Gayet-Ageron A, Prieto-Merino D, Ker K, et al. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018 Jan 13;391(10116):125-32. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5773762 http://www.ncbi.nlm.nih.gov/pubmed/29126600?tool=bestpractice.com It should be avoided if there are known hypercoagulable states.
Primary options
tranexamic acid: consult specialist for guidance on dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
tranexamic acid: consult specialist for guidance on dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
tranexamic acid
intravenous fluids
Treatment recommended for SOME patients in selected patient group
Early involvement of specialist multidisciplinary teams is recommended when cardiogenic shock is suspected (e.g., heart failure and critical care specialists, interventional cardiologists, and cardiac surgeons).[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com Aggressive fluid administration in the setting of cardiogenic shock may worsen the shock state and lead to the onset of acute pulmonary edema.
Intravenous fluids may be administered cautiously in the absence of signs of fluid overload (clinically determined by auscultation of the lungs to assess for rales or by chest radiography) in a patient with cardiogenic shock (e.g. small initial boluses [250mL] of crystalloid such as normal saline or Ringer lactate).[93]Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019 Apr 16;8(8):e011991. https://pmc.ncbi.nlm.nih.gov/articles/PMC6507212 http://www.ncbi.nlm.nih.gov/pubmed/30947630?tool=bestpractice.com
Ongoing management is guided by the continuous monitoring of organ perfusion and hemodynamics. Predicting the effect of further volume in any individual patient requires considerable clinical judgment, because no single, simple, and precise measure has been yet identified. Central venous pressure and ultrasonic parameters (central inferior vena cava diameter and collapsibility and aortic blood flow) are useful.[66]Nieminen MS, Böhm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. Eur Heart J. 2005 Feb;26(4):384-416. http://eurheartj.oxfordjournals.org/cgi/content/full/26/4/384 http://www.ncbi.nlm.nih.gov/pubmed/15681577?tool=bestpractice.com [79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90. http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com Commonly, systolic blood pressure of 90 mmHg or a mean arterial pressure of 65 mmHg is targeted during fluid volume resuscitation; infusion is stopped if concerns of volume overload develop (pulmonary rales or suspected pulmonary edema).
Fluid delivery is more important than the type of fluid (crystalloid or colloid).[71]Finfer S, Bellomo R, Boyce N, et al; the SAFE study investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004 May 27;350(22):2247-56.
http://www.nejm.org/doi/full/10.1056/NEJMoa040232#t=article
http://www.ncbi.nlm.nih.gov/pubmed/15163774?tool=bestpractice.com
[72]Roberts I, Blackhall K, Alderson P, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2011 Nov 9;(11):CD001208.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001208.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/22071799?tool=bestpractice.com
[73]Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018 Aug 3;(8):CD000567.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub7/full
http://www.ncbi.nlm.nih.gov/pubmed/30073665?tool=bestpractice.com
There is no evidence for any survival benefit of colloid fluids over crystalloid.[73]Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018 Aug 3;(8):CD000567.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub7/full
http://www.ncbi.nlm.nih.gov/pubmed/30073665?tool=bestpractice.com
[112]Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998 Mar 28;316(7136):961-4.
http://www.bmj.com/cgi/content/full/316/7136/961
http://www.ncbi.nlm.nih.gov/pubmed/9550953?tool=bestpractice.com
[113]Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999 Jan;27(1):200-10.
http://www.ncbi.nlm.nih.gov/pubmed/9934917?tool=bestpractice.com
[114]Akech S, Ledermann H, Maitland K. Choice of fluids for resuscitation in children with severe infection and shock: systematic review. BMJ. 2010 Sep 2;341:c4416.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2933356
http://www.ncbi.nlm.nih.gov/pubmed/20813823?tool=bestpractice.com
[115]Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014 Apr 10;370(15):1412-21.
http://www.nejm.org/doi/full/10.1056/NEJMoa1305727#t=article
http://www.ncbi.nlm.nih.gov/pubmed/24635772?tool=bestpractice.com
[  ]
How do colloids compare with crystalloids for fluid resuscitation in critically ill people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2307/fullShow me the answer[Evidence B]dcee690f-801f-4e69-9558-b7db51437fe3ccaBHow do colloids compare with crystalloids for fluid resuscitation in critically ill people? There is some evidence that balanced crystalloids may be preferable to normal saline in critically ill patients in intensive care, although other studies have found no difference.[76]Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
https://www.nejm.org/doi/full/10.1056/NEJMoa1711584?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://www.ncbi.nlm.nih.gov/pubmed/29485925?tool=bestpractice.com
[116]Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021 Aug 10;326(9):1-12.
https://jamanetwork.com/journals/jama/fullarticle/2783039
http://www.ncbi.nlm.nih.gov/pubmed/34375394?tool=bestpractice.com
In July 2021, the US Food and Drug Administration (FDA) issued safety labeling changes for solutions containing hydroxyethyl starch (HES) stating that HES products should not be used unless adequate alternative treatment is unavailable.[74]US Food and Drug Administration. Labeling changes on mortality, kidney injury, and excess bleeding with hydroxyethyl starch products. Jul 2021 [internet publication].
https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products
HES products are associated with adverse outcomes including kidney injury and death, particularly in critically ill patients and those with sepsis.[79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90.
http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com
[117]Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013 Feb 20;309(7):678-88.
https://jamanetwork.com/journals/jama/fullarticle/1653505
http://www.ncbi.nlm.nih.gov/pubmed/23423413?tool=bestpractice.com
In view of the serious risks posed to these patient populations, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency in February 2022 recommended suspending HES solutions for infusion in Europe.[75]European Medicines Agency. PRAC recommends suspending hydroxyethyl-starch solutions for infusion from the market. Feb 2022 [internet publication].
https://www.ema.europa.eu/en/news/prac-recommends-suspending-hydroxyethyl-starch-solutions-infusion-market-0
]
How do colloids compare with crystalloids for fluid resuscitation in critically ill people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2307/fullShow me the answer[Evidence B]dcee690f-801f-4e69-9558-b7db51437fe3ccaBHow do colloids compare with crystalloids for fluid resuscitation in critically ill people? There is some evidence that balanced crystalloids may be preferable to normal saline in critically ill patients in intensive care, although other studies have found no difference.[76]Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
https://www.nejm.org/doi/full/10.1056/NEJMoa1711584?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://www.ncbi.nlm.nih.gov/pubmed/29485925?tool=bestpractice.com
[116]Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021 Aug 10;326(9):1-12.
https://jamanetwork.com/journals/jama/fullarticle/2783039
http://www.ncbi.nlm.nih.gov/pubmed/34375394?tool=bestpractice.com
In July 2021, the US Food and Drug Administration (FDA) issued safety labeling changes for solutions containing hydroxyethyl starch (HES) stating that HES products should not be used unless adequate alternative treatment is unavailable.[74]US Food and Drug Administration. Labeling changes on mortality, kidney injury, and excess bleeding with hydroxyethyl starch products. Jul 2021 [internet publication].
https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products
HES products are associated with adverse outcomes including kidney injury and death, particularly in critically ill patients and those with sepsis.[79]Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: international consensus conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007 Apr;33(4):575-90.
http://www.ncbi.nlm.nih.gov/pubmed/17285286?tool=bestpractice.com
[117]Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013 Feb 20;309(7):678-88.
https://jamanetwork.com/journals/jama/fullarticle/1653505
http://www.ncbi.nlm.nih.gov/pubmed/23423413?tool=bestpractice.com
In view of the serious risks posed to these patient populations, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency in February 2022 recommended suspending HES solutions for infusion in Europe.[75]European Medicines Agency. PRAC recommends suspending hydroxyethyl-starch solutions for infusion from the market. Feb 2022 [internet publication].
https://www.ema.europa.eu/en/news/prac-recommends-suspending-hydroxyethyl-starch-solutions-infusion-market-0
If there is acute inferior (right coronary artery) occlusion, or right ventricular infarct, hypotension is initially (before thrombectomy) corrected with careful fluid administration to improve right ventricular filling and overall cardiac output.[77]Inohara T, Kohsaka S, Fukuda K, et al. The challenges in the management of right ventricular infarction. Eur Heart J Acute Cardiovasc Care. 2013 Sep;2(3):226-34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3821821 http://www.ncbi.nlm.nih.gov/pubmed/24222834?tool=bestpractice.com
nitroglycerin
Treatment recommended for SOME patients in selected patient group
Patients with cardiogenic shock with pulmonary edema and relatively stable blood pressure (with systolic blood pressure >90 mmHg) may benefit from afterload reduction with intravenous nitroglycerin.
Primary options
nitroglycerin: 5-20 micrograms/minute intravenous infusion initially, titrate according to effect (improved cardiac function and decreased pulmonary edema symptoms), maximum 200 micrograms/minute
These drug options and doses relate to a patient with no comorbidities.
Primary options
nitroglycerin: 5-20 micrograms/minute intravenous infusion initially, titrate according to effect (improved cardiac function and decreased pulmonary edema symptoms), maximum 200 micrograms/minute
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
nitroglycerin
temporary mechanical circulatory support (MCS)
Treatment recommended for SOME patients in selected patient group
Temporary MCS devices (e.g., extracorporeal membrane oxygenation or intra-aortic balloon pump) should be considered in patients with persistent cardiogenic shock maintained despite inotropic therapy.[3]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726. https://academic.oup.com/eurheartj/article/42/36/3599/6358045 http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com [4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com Temporary MCS in appropriately selected candidates should be initiated as soon as possible with sufficient support to fully reverse the potential hemometabolic consequences of shock. If there is a delay to sufficient early support with temporary MCS, worsening end-organ perfusion and metabolic derangements can make future attempts difficult.[83]Geller BJ, Sinha SS, Kapur NK, et al. Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2022 Aug 9;146(6):e50-68. https://www.doi.org/10.1161/CIR.0000000000001076 http://www.ncbi.nlm.nih.gov/pubmed/35862152?tool=bestpractice.com The hemodynamic benefits of the specific devices vary, and few head-to-head randomized comparisons exist. Vascular, bleeding, and neurologic complications are common to MCS devices, and the risk of such complications should generally be considered in the calculation to proceed with such support.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com MCS requires specialist multidisciplinary expertise for implantation and management.[3]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726. https://academic.oup.com/eurheartj/article/42/36/3599/6358045 http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com The escalation of either pharmacologic and mechanical therapies should be considered in the context of multidisciplinary teams of heart failure and critical care specialists, interventional cardiologists, and cardiac surgeons.[4]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-421. https://www.doi.org/10.1016/j.jacc.2021.12.012 http://www.ncbi.nlm.nih.gov/pubmed/35379503?tool=bestpractice.com [83]Geller BJ, Sinha SS, Kapur NK, et al. Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2022 Aug 9;146(6):e50-68. https://www.doi.org/10.1161/CIR.0000000000001076 http://www.ncbi.nlm.nih.gov/pubmed/35862152?tool=bestpractice.com

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer