Approach
The clinical manifestations of SARS are non-specific and mimic other causes of respiratory infection.[16] However, a combination of epidemiological and clinical features increase the likelihood of a diagnosis of SARS, which is confirmed with viral testing. Maintaining a high index of suspicion, in case of a re-emergence of SARS-CoV infection, is essential for the early diagnosis of the disease.
Although haematological and radiological findings are only suggestive of a diagnosis in a suspected case of SARS, these investigations are indicative of disease prognosis and are essential for the follow-up of treatment.
Clinical history
The aim of the history is to demonstrate epidemiological and clinical features that will aid diagnosis.[21]
It should include questioning on the following epidemiological factors:
History of recent travel, within 10 days of the onset of symptoms, to a foreign or domestic location with documented or suspected recent transmission of SARS: raises suspicion of the infection.[21]
History of close, prolonged contact with an individual suspected of or documented as being infected with SARS-CoV.[22]
History of exposure to anyone with an unexplained respiratory illness who has recently travelled to a country affected by SARS.[21]
History of contact with contaminated materials: of particular importance in relation to laboratory workers.[24]
The chronology of symptoms is also important.
Prodromal symptoms are similar to those of other viral infections, with myalgia, malaise, and headache.
These progress to the early symptoms of fever with associated chills (rapid onset and persistent), cough (usually non-productive and develops 2 to 7 days from the onset of symptoms), and sore throat.
Later symptoms include dyspnoea (develops 8 to 12 days from the onset of symptoms), watery diarrhoea (occurs in 20% to 25% of cases in the second week), chest pain, and pleurisy.[4][24]
Other less common symptoms include nausea and vomiting, abdominal pain, rhinorrhoea, arthralgia, sputum production, dizziness, and seizure.
The typical febrile response may be absent in older adult patients, who may present with non-specific symptoms such as malaise, loss of appetite, or delirium, or even an episode of a fall with an associated fracture.[4] Infants and children present with milder symptoms and associated rhinorrhoea in 50% of cases.
Physical examination
On examination, the patient may be febrile with a temperature of ≥38°C (100.4°F), with rigors, shortness of breath, tachypnoea, tachycardia, and cyanosis.[4] Auscultation of the chest may reveal inspiratory crackles, rales, and/or bronchial breathing.
Less common findings in atypical cases include altered mental status, confusion, and delirium.
Initial investigations
First-line evaluation of a patient with suspected SARS should include routine laboratory tests, cultures, and a chest x-ray, with pulse oximetry and ABG measurement necessary in patients with respiratory distress and cyanosis.
Blood tests
FBC: leukopenia is common, with lymphopenia reported in 98% of patients early in the disease.[3] Lymphopenia is due to reduced CD4 and CD8 cell counts. Thrombocytopenia is found in the presence of disseminated intravascular coagulation.
Liver function tests (LFTs): mild elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) has been reported in 23% to 50% of SARS patients, although this result shows low specificity for diagnosis of the disease.[19]
Lactate dehydrogenase: elevated.
Creatine kinase: elevated.
Blood and sputum cultures
All patients with signs of severe infection should receive blood and sputum cultures to rule out other causes of a lower respiratory tract infection, such as community-acquired pneumonia, especially those without a typical epidemiological history for SARS.
Blood cultures would be negative for a bacterial infection, and the sputum culture would not show growth of Streptococcus pneumoniae or other infecting bacteria in SARS.
Tests for influenza virus
Nasopharyngeal virus culture and direct immunofluorescent antibody staining would be negative for influenza A and influenza B viruses in SARS.
Chest x-ray
About 20% to 25% of cases have a normal chest x-ray on presentation.[24][25]
Examined for unilateral or bilateral infiltrates in the peripheries of the lower zones. Infiltrates manifest as patchy, confluent, or diffuse consolidation, or nodular shadowing.
Cavitation, hilar lymphadenopathy, and pleural effusion are not typically seen.[26]
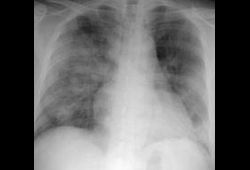
Pneumomediastinum and pneumothorax often occur with assisted ventilation.[27][Figure caption and citation for the preceding image starts]: Chest x-ray of a SARS patient showing multifocal opacitiesKetai L, et al. J Thorac Imaging. 2006;21:276-283; used with permission [Citation ends].
Further investigations
Pulse oximetry
Indicated in patients with respiratory distress and cyanosis.
Reveals a low oxygen saturation (SpO₂ <90%).
ABG
Performed when the SpO₂ measured with pulse oximetry is <90%.
Reveals low partial oxygen pressure.
Coagulation screen
Indicated in patients with spontaneous bleeding.
Reveals a prolonged activated partial thromboplastin time (aPTT) and raised D-dimers.
High-resolution CT (HRCT)
Imaging of the thorax with HRCT should be undertaken in those with a normal chest x-ray on presentation and a high suspicion of SARS for the detection of lung opacities.
Reveals ground glass opacities with interlobular septal thickening and, in some cases, subpleural consolidation.
Abnormal in 67% of patients with an initially normal chest x-ray.[28]
Specific viral testing
Reverse-transcription polymerase chain reaction (RT-PCR)
Detecting SARS-CoV-specific RNA directly using an RT-PCR assay is the mainstay of laboratory diagnosis and should be ordered in all suspected cases immediately.
For a positive diagnosis based on PCR testing, the patient must have either two positive clinical specimens from different anatomical sites or positive specimens from the same site on two separate occasions.[29][30]
Multiple specimen sources should be obtained. During the first week, nasopharyngeal, oropharyngeal, and serum/plasma specimens should be tested and, following this, nasopharyngeal, oropharyngeal, and stool specimens should be sampled.
Preferred samples are respiratory tract specimens (nasopharyngeal aspirate or throat swab) obtained in viral transport media. Stool specimens or whole blood samples in ethylene diamine tetra-acetic acid (EDTA) are also appropriate.
Nasopharyngeal specimens, which are often negative during the first week of infection, have the highest positivity rates in the second week of illness, peaking at approximately day 10.
The positivity rates on urine, nasopharyngeal aspirate, and stool specimen have been reported to be 42%, 68%, and 97%, respectively, on day 14 of illness.[11]
The sensitivity of these tests, which is highly dependent on the type of specimen and increases the later the sample is collected after the onset of symptoms, ranges from 83.3% to 100%. The specificities range from 94% to 100%.[31]
Respiratory and stool specimens have the highest yield (between 80% and 90%) 10 to 14 days into the course of the disease.[32]
Improved methods of specimen collection and real-time RT-PCR assays have improved the sensitivity of testing during the first few days of illness.[33]
Quantitative measurement of blood SARS-CoV RNA with the technique of real-time RT-PCR has a detection rate of 80% as early as day 1 of the disease, with a subsequent drop to 45% on day 14.[33]
Serological testing for SARS-CoV-specific antibodies
Tested using an immunofluorescent antibody assay (IFA) or enzyme-linked immunosorbent assay (ELISA).
Useful for epidemiological surveillance and retrospective diagnosis.
Serum specimens should be collected when the diagnosis is first suspected and at later times if indicated.
Seroconversion usually occurs 1 to 4 weeks (>90% after day 28) after the onset of symptoms, with an antibody response (4-fold increase in SARS-CoV antibody) occasionally detected during the first week of illness, likely to be detected by the end of the second week of illness, but not normally detected until >28 days into the illness.[30][34]
Viral culture
Not recommended for routine detection.[30]
Lacks sensitivity of RT-PCR.
Given the potential risk of transmission, growth of the SARS-CoV virus should be restricted to biosafety level III (or IV) laboratories.[34]
Rapid immunoswab assay for SARS-CoV detection
An emerging diagnostic test.
The key feature of this simple immunoswab diagnostic assay is its ability to detect the presence of the SARS-CoV antigen (nucleocapsid protein) within 45 to 60 minutes following availability of the body fluid samples.[35]
Monoclonal antibodies
An emerging diagnostic test.
Five monoclonal antibodies against the recombinant nucleocapsid protein of SARS-CoV were developed by hybridoma technology.
Potentially ideal candidates for developing early and sensitive diagnostic assays for SARS-CoV.[36]
How to obtain an arterial blood sample from the radial artery.
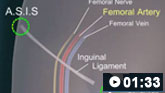
How to perform a femoral artery puncture to collect a sample of arterial blood.
Use of this content is subject to our disclaimer