Approach
A poison centre should be contacted as soon as a toxic ingestion is suspected. Management of poisoned patients should proceed with the consultation of a specialist familiar with their care. Availability and contact with poison centres may differ between countries and regions, and carers and healthcare providers should keep a record of the local contact number.
Symptomatic patients, and those with deliberate or high-risk ingestions and ingestions requiring blood level determination (e.g., paracetamol, salicylates, warfarin) require assessment in the emergency department. Those who are symptomatic require urgent intervention with establishment of an airway, assessment of ventilatory effectiveness in both oxygenation and elimination of carbon dioxide, and maintenance of circulation as shown by a capillary refill of <2 seconds, an adequate BP, and end-organ perfusion. The mainstay of management is supportive care. The need for any further management (e.g., GI decontamination) should be discussed with a poison centre.
Patients presenting within 1 hour of ingestion may benefit from GI decontamination methods to decrease absorption and enhance toxin elimination, but their use should not be routine and is best discussed with an expert experienced in the management of poisoning in children or a poison control centre.
Patients may require antidotes for specific poisonings.[38][39] If either the antidote or a laboratory test required to guide its use are not available, the child should be transferred to a unit that has the ability to provide the antidote and perform the monitoring. There are a few ingestions that require extra-corporeal drug removal to achieve complete elimination.
Prompt diagnosis and treatment lead to a good outcome in most cases.[40][41][42][43]
In the US, the majority (67%) of human exposures reported to poison control centres in 2021 were managed outside of a healthcare facility (generally in the patient's own home) with phone follow-up from the poison control centre.[1]
Asymptomatic patients
All children suspected of having a toxic ingestion require observation. The minimum observation period is typically 6 hours, but children who have ingested a substance that carries a high risk of delayed toxicity require observation for 24 hours.
High-risk ingestions include acetonitrile, antimalarials (chloroquine or hydroxychloroquine), antiarrhythmics, antipsychotics, benzocaine, beta-blockers, calcium-channel blockers, camphor and other essential oils, cholinesterase inhibitors, clonidine and alpha-2 agonists, cocaine, tricyclic antidepressants, colchicine, bupropion, ethylene glycol, lindane, diphenoxylate/atropine, methanol, salicylates, opioids (especially methadone and buprenorphine), sulfonylureas, and theophylline.[40][41][43][44][45]
Deliberate or high-risk ingestions should be monitored in hospital. In addition, any history of cyanide exposure should be referred to hospital. Those with warfarin ingestions should be referred for an INR and monitoring.
Paediatric patients with all non-accidental ingestions, or any suspicion of cough or cold/antipyretic/analgesic accidental ingestion, should be referred to hospital to have a paracetamol and salicylate level check, as the child may be asymptomatic on initial presentation.
Emergency management
Airway and breathing
Any medication that markedly reduces mental status can lead to loss of the airway and airway reflexes. Several medications depress the respiratory drive, including sedative-hypnotics, opioids, central alpha-agents such as clonidine, and antipsychotics. Some medications can increase oral and bronchial secretions (cholinergics and antipsychotics); the presence of a gag reflex in these patients may not be sufficient to protect the airway.
Appropriate airway positioning to maintain patency is important, remembering that a child up to toddler age has a significantly larger head and smaller mouth and airway than adults. Use of airway adjuncts such as oral and nasopharyngeal airways may be necessary. Use of these adjuncts may increase the risk of vomiting.
Intubation and admission to the intensive care unit may be required in severe cases. Intubation may increase the risk of vomiting.
Ingestion of corrosive materials such as strong acids and bases should lower the threshold for intubation, especially if signs of stridor are present. There should also be a low threshold to intubate an unresponsive poisoned child with evidence of increased airway secretions or hypoventilation. In cases of multiple drug ingestion, depressed respiratory drive and respiratory acidosis can exacerbate the toxic effects of other medications, including salicylates, toxic alcohols and glycols, and sodium-channel agents such as tricyclic antidepressants. Metabolic acidosis causes hyperpnoea and/or tachypnoea, thereby increasing the tidal volume to a level sufficient to compensate for the acidosis. Intubation should be undertaken with extreme caution in these patients because any airway interventions that decrease the minute ventilation will lead to a sudden and catastrophic decrease in serum pH. If intubation is required (usually due to respiratory fatigue), care must be taken to maintain the respiratory rate and volume at the same level as was present before intubation or the onset of fatigue.
Most of the morbidity and mortality from toxic ingestions is due to issues of airway and breathing, so good airway management is crucial. Oxygen saturation should be monitored continuously, and capillary blood gas or arterial blood gas measurements taken regularly. Ventilation in intubated patients should be assessed using non-invasive end-tidal carbon dioxide monitoring.
Circulation
Hypotension: many overdoses are negative inotropes, negative chronotropes, or peripheral vasodilators resulting in hypotension. Children maintain their physiological parameters far better than adults but then deteriorate suddenly without any reserves. Symptomatic patients require intravenous fluids to aid in dilution and elimination of the toxin. Hypotension and decreased perfusion should be treated with fluid boluses (20 mL/kg) with an isotonic fluid such as normal saline and Ringer lactate. If BP cannot be maintained using fluids alone, vasopressors should be considered. In most overdoses, the pressor of choice is an alpha agonist such as noradrenaline (norepinephrine) or a mixed agent such as adrenaline (epinephrine). However, these pressors do not produce a response in beta-blocker or calcium-channel blocker toxicity; a lack of response should prompt consideration of these toxicities, which must be managed using the appropriate antidotes.
Hypertension: sympathomimetic toxicity causes a marked elevation in BP. Benzodiazepines are the specific antidotes for this type of poisoning. However, vasodilators may also be required to lower BP if BP rises above a critical threshold (as defined by local protocols) or if there is clinical evidence of end-stage organ dysfunction (cardiac, renal, or CNS abnormalities). Vasodilators to consider include glyceryl trinitrate, nitroprusside, phentolamine, and hydralazine.
Evidence of adequate perfusion should be repeatedly checked and include capillary refill as well as measures of end-organ perfusion such as urine output, mental status, and lactate production. Cardiovascular instability may also require central monitoring of cardiac output and systemic vascular resistance, by either invasive or non-invasive monitoring. Fine adjustment of pressors and inotropes usually requires invasive monitoring.
Gastrointestinal decontamination
Activated charcoal
Patients who are awake and alert with an intact airway and no evidence of hydrocarbon or caustic ingestion may be given a dose of activated charcoal (if possible within 1 hour of ingestion). However, there is no clear evidence to support that this intervention changes clinical outcomes of poisoned patients.[46]
Activated charcoal is contra-indicated in hydrocarbon and caustic ingestions, and is ineffective in lithium and some other ingestions.
Drugs that are absorbed by charcoal include theophylline, carbamazepine, quinine/quinidine, dapsone, thallium, and phenobarbital. Other medications that are partially absorbed include almost all anticonvulsants and salicylates. Drugs that are not absorbed by charcoal include heavy metals (except thallium), salts, lithium, ethanol, methanol, caustics, hydrocarbons, and ethylene glycol.
Multiple-dose activated charcoal may also be useful in enhancing drug elimination for phenobarbital, quinine, dapsone, theophylline, or carbamazepine. However, a randomised controlled trial in 2002 noted no outcome difference in the use of multiple-dose activated charcoal.[47]
Discussion with a regional poison control centre prior to the use of activated charcoal is recommended.
Whole bowel irrigation
Possible indications include ingestions of sustained-release or enteric products; multiple drug packets (body packing); and large ingestions of iron, potassium, or lithium. There is no firm outcome evidence to suggest that this technique improves clinical outcomes.[48]
The procedure involves infusion of a polyethylene glycol electrolyte solution at 20-40 mL/kg/hour (maximum rate of 1-2 L/hour) via a nasogastric tube until effluent is clear.
It is recommended that the use of whole bowel irrigation be discussed with a regional poison control centre.
Gastric lavage is rarely, if ever, required. There is poor evidence that it changes outcome, and there are significant side effects associated with it. It should only be considered by personnel who are properly trained in its use and contraindications.[49]
Ipecac syrup, cathartics, and laxatives should not be used in poisoned patients. There is no evidence to suggest that these agents improve outcomes following toxic ingestion.[50][51]
Supportive care
Electrolyte disturbances
Potassium, sodium, calcium, and magnesium should be replaced intravenously as needed, and serum electrolytes monitored regularly. Patients with a long QT interval should also receive magnesium supplementation to prevent torsades de pointes.
Sedation
Sedation when intubated is important and is best handled with opioid analgesia and benzodiazepine/propofol sedation, because these have the least effect on cardiovascular parameters and neurotransmitters. Sedation with dexmedetomidine in the poisoned patient lacks outcome data and may worsen hypotension and bradycardia.
Temperature control
External cooling and depression of muscular activity are extremely important in preventing hyperthermia, metabolic acidosis, and secondary rhabdomyolysis. Non-depolarising paralysis may be required to halt heat and acid production.
Care must also be taken not to let a patient become hypothermic. This may happen with some sedatives including baclofen and carisoprodol.
Seizures
Should be controlled using GABA agonists such as benzodiazepines or barbiturates. Sodium-channel anticonvulsants such as phenytoin should be avoided because patients with toxic ingestions do not respond to these agents.
Nutrition
In patients who are hypermetabolic, even minimal stress can overwhelm the glycogen stores and precipitate ketogenesis. This is especially true in paediatric ingestions of ethanol. Calorie and nutrient provision to maintain nutritional status is therefore important.
Specific antidotes
Opioid toxicity
The antidote is naloxone. Treatment is indicated if there is respiratory depression or decreased mental status, provided that the patient is not intubated.[52]
Paracetamol overdose
The antidote is acetylcysteine. Treatment of a single acute ingestion is guided by a paracetamol treatment graph. The graph used varies between countries, and physicians should consult the local paracetamol overdose protocols. For example, in the UK, a treatment graph that covers normal and high-risk patients is used, whereas in the US, the Rumack-Matthew nomogram is used, and a similar guideline is used in Australia and New Zealand.[36][37][Figure caption and citation for the preceding image starts]: Paracetamol treatment graph. This consists of a normal treatment line, which connects 1.32 mmol/L (200 mg/L) at 4 hours and 0.04 mmol/L (6.25 mg/L) at 24 hours, and a high-risk treatment line for those at increased risk of liver damage, which connects the points at 50% of the plasma paracetamol concentrations of the normal line. Patients should be treated if their plasma paracetamol concentration is above their appropriate treatment lineWith permission of Professor P.A. Routledge, Therapeutics and Toxicology Centre, Cardiff University [Citation ends].
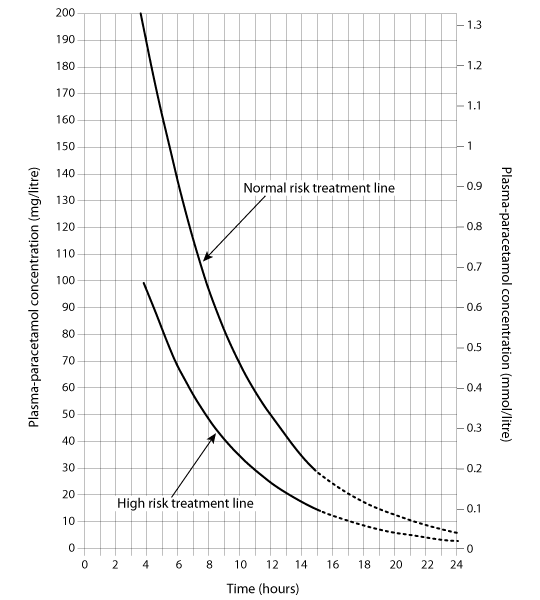
If the child presents >8 hours after ingestion or has had repeated supratherapeutic ingestions, the first dose of acetylcysteine should be given immediately.[53][54][55]
Patients with repeated supratherapeutic ingestions should be treated if symptomatic (nausea/vomiting) or have elevated liver function tests regardless of paracetamol levels.
If there are any concerns regarding initiating acetylcysteine or its route, dosing, or duration of therapy, consultation with an appropriate specialist is recommended.
Beta-blocker toxicity
Treatment is indicated if there is bradycardia and hypotension. Intravenous glucagon should be commenced.[52]
If there is no response to glucagon, high-dose insulin (hyperinsulinaemia-euglycaemia therapy) should be used instead.[52] This involves administration of pharmacological doses of intravenous insulin with co-administration of dextrose to maintain blood glucose levels. Specialist advice should be sought for details of dosing.
Calcium-channel blocker toxicity
Treatment is indicated if there is symptomatic bradycardia and hypotension. Initial treatment is with calcium chloride.[52]
If the response is still inadequate, hyperinsulinaemia-euglycaemia therapy can be used.[56] The protocol involves administration of an intravenous insulin loading dose, followed by an infusion with dose increases every 15 to 30 minutes as required. Blood glucose is maintained using concurrent intravenous supplementation. Specialist advice should be sought for details of dosing.
Sodium-channel blocker toxicity
All children with reported ingestion of a sodium-channel blocker greater than the maximal single therapeutic dose for children should be observed in hospital for a minimum of 6 hours.
In patients in whom there is widening of the QRS >100 milliseconds on ECG, serum alkalinisation with intravenous sodium bicarbonate should be started immediately.[57] Alkalinisation cannot be achieved if the patient is hypokalaemic, and appropriate potassium replacement should also be given as needed to maintain a serum potassium of >3.8 mEq/L.
The sodium bicarbonate infusion should be continued for 12 hours and then stopped. If widening of the QRS returns, a second 12-hour infusion should be given, and this procedure should be repeated until the QRS remains <100 milliseconds at normal pH.
Alkalinisation is reserved for QRS >100 milliseconds. There are no data to suggest that prophylactic alkalinisation prevents deterioration. The grey area is when the child is wide awake and alert with a QRS >100 milliseconds. Because it is extremely unusual to have abnormal conduction without mental status changes, if there is a question, then the physician can consider a trial of an intravenous push of 1 to 2 mEq/kg of sodium bicarbonate. If the QRS narrows, then therapy can be continued. If not, then the widened QRS is likely to be the patient’s pre-morbid ECG. Specialist advice should be sought if this class of poisoning is suspected.
Salicylate or phenobarbital toxicity
Treatment of salicylate poisoning involves urine alkalinisation, which is achieved by the administration of intravenous sodium bicarbonate.[58] Alkalinisation cannot be achieved if the patient is hypokalaemic, and appropriate potassium replacement should also be given as needed to maintain a serum potassium at >3.8 mEq/L. The aim of urinary alkalinisation is a urine pH >7.5, which should be maintained until the salicylate level is <1448 to 1810 micromol/L (<20 to 25 mg/dL or 200 to 250 micrograms/mL) with resolution of anion gap metabolic acidosis. Urine alkalinisation may be considered for phenobarbital toxicity but should be discussed with a poison control centre.
All children with salicylate ingestions need to be evaluated with both acid-base status and level, because the tissue end-organ effects determine toxicity and are extremely pH-dependent. A large ingestion is suggested by a salicylate level >1448 to 2172 micromol/L (>20 to 30 mg/dL or 200 to 300 micrograms/mL) within 1 to 2 hours of ingestion, and immediate action should be taken to administer urine alkalinisation. As the hyperpnoea of salicylate toxicity, which is the first sign of symptoms, is often missed, a conservative approach would be to start urinary alkalinisation in any patient with a level >2172 to 2534 micromol/L (>30 to 35 mg/dL or 300 to 350 micrograms/mL) and who may seem 'asymptomatic', or any level >1448 micromol/L (>20 mg/dL or 20 micrograms/mL) with any acid-base disturbance (gap >14 mmol/L or 14 mEq/L). Specialist consultation should be sought with suspected salicylate poisoning in order to discuss whether haemodialysis is indicated.
Salicylate levels should be checked 4 to 6 hours after stopping the sodium bicarbonate infusion, because a rebound rise in salicylate level may occur.
The use of carbonic anhydrase inhibitors is not recommended as it may worsen systemic toxicity.
Phenobarbital elimination is enhanced by multi-dose activated charcoal; however, evidence regarding improved clinical outcomes with its use is less clear and should be discussed with a poison control centre.
Benzodiazepine toxicity
Treatment with flumazenil may be indicated if there is respiratory depression or failure, but extreme caution should be taken with patients who are taking benzodiazepines chronically or are benzodiazepine tolerant.[52] Seizures caused by flumazenil may be unresponsive to benzodiazepines.
Methanol or ethylene glycol toxicity
Treatment is indicated if there is good evidence of ingestion or any evidence of end-organ toxicity (visual disturbance, acidosis, hypotension, acute kidney injury), or any symptoms consistent with reported ingestion until it can be confirmed. Ingestion of 10 mL of 100% methanol in a 10-kg child will lead to a level >12.5 mmol/L (>40 mg/dL or 400 micrograms/mL), so if there is a good suggestive history of ingestion, the physician may consider initiating treatment while waiting for methanol levels. The first-line treatment is fomepizole.[59] However, oral ethanol loading or an ethanol infusion can also be used if fomepizole is unavailable. Haemodialysis may be required and should be discussed with a regional poison control center or specialist experienced in the management of poisoning in children.
Cholinesterase inhibitor toxicity
Antidote is indicated if there is symptomatic bradycardia. Intravenous atropine alone is sufficient in mild to moderate cases. Severe cases also require intravenous pralidoxime.[60]
Cyanide toxicity
Treatment with hydroxocobalamin, or combination treatment with nitrites followed by thiosulfate, is indicated if there is coma, seizures, or metabolic acidosis.[61][62]
Warfarin toxicity
Fresh frozen plasma should be given in all patients with active bleeding and an INR >5. Vitamin K can be given if INR >5-10.
Digoxin toxicity
The antidote is digoxin-specific antibody fragments (digoxin immune Fab) and should be given if there is symptomatic bradycardia, hypotension, hyperkalaemia, or any cardiac arrhythmia.[52]
Sulfonylurea toxicity
Treatment with glucose infusions in combination with intravenous or subcutaneous octreotide is indicated if there is recurrent hypoglycaemia <2.2 mmol/L (40 mg/dL) or if hypoglycaemia is symptomatic.[63]
Heavy metal toxicity
Treatment with an appropriate chelating agent may be indicated in a symptomatic patient.
Haemodialysis and haemoperfusion
The options are either high flux haemodialysis, which is preferred, or haemoperfusion across a charcoal canister. The indications for extracorporeal treatment are:
Ingestion of agents amenable to extracorporeal treatment. Examples of drugs amenable to haemodialysis include salicylates, lithium, methanol, ethylene glycol, and theophylline. Haemoperfusion may be considered for carbamazepine, valproate, and theophylline.
The decision to utilise extracorporeal treatment should take into account toxin levels, clinical evidence of severe toxicity or significant end-organ toxicity, and poor clinical response to other standard therapies.
Consideration of extracorporeal treatment should be discussed with a regional poison centre.
Peritoneal dialysis is not a useful therapy in poisonings at present. Continuous veno-venous haemofiltration is an alternative method that is being investigated.
Online treatment information
A number of online resources are available that provide information on the treatment of a wide range of known poisonings:
Use of this content is subject to our disclaimer