Approach
The diagnostic work-up of patients with suspected Budd-Chiari syndrome (BCS) should include not only diagnostic confirmation of hepatic vein occlusion but also the identification of the underlying pro-coagulant haematological cause. The diagnosis of BCS is suggested by clinical suspicion and further confirmed by imaging techniques.
Clinical evaluation
BCS should be suspected in patients with a personal or family history of thrombophilia who present with the classic triad of abdominal pain (specifically right upper quadrant abdominal pain), ascites, and hepatomegaly.
Patients may also have or develop splenomegaly and gastrointestinal bleeding as a consequence of the underlying myeloproliferative disease, portal vein thrombosis, or as a sequelae of cirrhosis (with portal hypertension) that develops in the chronic form of BCS.
Careful cardiovascular exam and the absence of the hepatojugular reflux are required to rule out cardiac conditions with similar presentation, such as tricuspid regurgitation, constrictive pericarditis, and right atrial myxoma.
Leg oedema and dilated venous collaterals on the trunk indicate inferior vena cava (IVC) compression or thrombosis.[9]
The fulminant form of BCS is uncommon. Patients rapidly develop jaundice, hepatic encephalopathy, renal failure, and coagulopathy. The liver is enlarged and tender.[10] The symptoms of the fulminant form of BCS are similar to those in acute hepatitis.
Imaging
Colour and pulsed Doppler ultrasonography is considered the first-line investigation for patients with suspected BCS (sensitivity and specificity >85%).[33] The combination of specific findings (thrombosis, stenosis, fibrotic cord, or insufficient re-canalisation of hepatic and/or caval veins) and caudate lobe hypertrophy has the highest predictive value to identify patients with BCS.
Abdominal CT and/or MRI are then used to visualise the hepatic veins and the whole length of the IVC and demonstrate occlusion of hepatic veins, IVC, or both.[1] They also offer better visualisation of liver parenchymal abnormalities and necrotic areas that differentiate the acute form of BCS from chronic disease, and can exclude alternative diagnoses.[34] MRI is more accurate than CT and allows optimal delineation of venous obstruction for therapeutic decisions.[35]
Hepatic venography (invasive) is considered the definitive diagnostic tool. The presence of a spider web pattern on hepatic venography confirms the diagnosis of BCS.[1] It is needed to determine the extent of thrombosis and a measure of the portacaval venous pressure gradient, which is often useful before portacaval shunting procedures. It also facilitates transjugular liver biopsy, which can be helpful to assess presence of cirrhosis and exclude alternative or concomitant liver diagnoses. Hepatic venography is carried out when the suspicion of BCS is high, even in the absence of typical findings on other radiological imaging. It should also be performed when percutaneous or surgical shunting is considered.
Contrast-enhanced three-dimensional magnetic resonance angiography demonstrates vascular structures, both in the liver and in the upper abdomen, in various planes, and also yields information concerning the liver parenchymal status.[36] It is more useful than other imaging techniques in evaluating treatment options, and in assessing therapeutic effects in the follow-up period.[37] It is done when it is impossible to cannulate the hepatic veins during hepatic venography, and in patients with renal insufficiency, as venography usually requires the use of considerable amounts of iodine-containing contrast medium.
Secondary BCS is caused by external compression or invasion of the venous lumen by abscess, tumours, or cysts. Liver imaging allows recognition of the lesions causing secondary BCS.[38][Figure caption and citation for the preceding image starts]: Hepatic venogram demonstrating 'spider web' and thrombus in the inferior vena cavaLiver Transplantation Journal. 2006;12:S21-S22; reprinted with permission of John Wiley & Sons, Inc. [Citation ends].
Tests for underlying haematological disorders
A full blood count should be performed to look for evidence of a primary myeloproliferative disorder (MPD) such as polycythaemia vera, essential thrombocytosis, or myelofibrosis. Consultation with a haematologist is warranted.[1] About 80% of patients with BCS and with an MPD harbour JAK2(V617F) mutation.[3][39] One meta-analysis showed the pooled prevalence of JAK2(V617F) mutation to be 37% in patients with BCS. After pre-existing MPD was excluded, the pooled prevalence was decreased to 26%. This mutation was found to be more specific to thrombosis in splanchnic areas and strongly associated with the development of MPD in these patients.[40]
JAK2 mutation testing has facilitated the diagnosis of an occult type of MPD. Testing for JAK2 mutation is done when the diagnosis of an MPD proves to be difficult: for example, if the typical changes in peripheral blood, such as high levels of haemoglobin, platelets, or white blood cells, are absent; or if conventional diagnostic criteria are not met. (Reference standards for a diagnosis of MPD include splanchnic vein thrombosis and clusters of abnormal megakaryocytes in bone marrow biopsy, combined with endogenous erythrocyte colony formation and the combination of marked splenomegaly and platelet count >200 x 10⁹/L [>200 x 10³/microlitre]).[41] However, in cases of suspected MPD that are JAK2-negative, bone marrow biopsy is required. Testing for JAK2 mutation and bone marrow biopsy have replaced formerly used tests using cultures of erythroid progenitors to demonstrate endogenous colonies.
Thrombophilia screening is usually done if liver disease is observed in patients with known thrombophilia, and, because a combined aetiology is found in at least 25% of the patients, identification of a single cause should not preclude investigation of other aetiological factors. Tests include:[1]
Genetic testing for factor Leiden V, prothrombin factor, methylenetetrahydrofolate reductase mutation
Serum levels of protein C and S, antithrombin III, and homocysteine; lupus anticoagulant, anti-beta2 glycoprotein-1 antibodies, anticardiolipin antibodies (antiphospholipid syndrome)
Flow cytometry for CD55- and CD59-deficient blood cells (paroxysmal nocturnal haemoglobinuria). [Figure caption and citation for the preceding image starts]: Tests for prothrombotic conditions in patients with Budd-Chiari syndromeFrom the personal collection of Soha Saoud Abdel Moneim, MD, PhD and Vijay H. Shah, MD; used with permission [Citation ends].
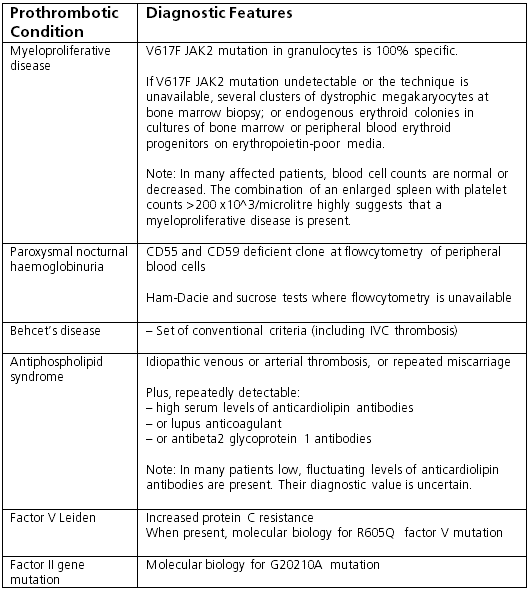 [Figure caption and citation for the preceding image starts]: Tests for prothrombotic conditions in patients with Budd-Chiari syndromeFrom the personal collection of Soha Saoud Abdel Moneim, MD, PhD and Vijay H. Shah, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Tests for prothrombotic conditions in patients with Budd-Chiari syndromeFrom the personal collection of Soha Saoud Abdel Moneim, MD, PhD and Vijay H. Shah, MD; used with permission [Citation ends].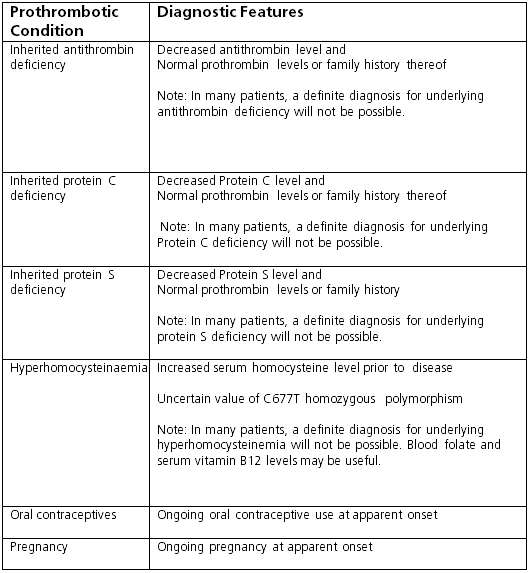
Other investigations
Liver function test results are non-specific but almost always elevated to some extent. Significant elevation in serum aspartate and alanine aminotransferase levels, to 5 times or more above the upper limit, is usually seen in fulminant and acute forms of BCS. This can also be seen in patients with acute hepatitis due to different aetiologies. Levels of alkaline phosphatase and bilirubin may also increase along with a decrease in serum albumin. Severe impairment of prothrombin time occurs in fulminant form.[1]
A high serum-ascites albumin gradient (SAAG) in combination with a total ascitic protein >25 g/L (>2.5 g/dL) supports a diagnosis of non-cirrhotic BCS, although the ascitic protein may be low in chronic BCS with concomitant cirrhosis. However, high SAAG with high protein ascites may also be seen in cardiac causes of ascites.[42]
Renal function and coagulation studies should be performed as baseline, and may be abnormal in fulminant BCS.
Liver biopsy is not required to confirm the diagnosis as the disease is heterogeneous, giving rise to high risk of sampling error.[43] Liver biopsy is not recommended as standard in children, adolescents, or young adults.[44] It is needed only in the small subset of patients in whom BCS is related to pure thrombotic involvement of the small hepatic veins, where major hepatic veins and IVC appear patent at imaging.[1][2] It is also useful for ruling out other processes such as veno-occlusive disease and cirrhosis of other aetiologies. Liver biopsy should be carried out at the same time as angiographic investigation to confirm the diagnosis (if possible) and assess liver reserve and potential reversibility of liver injury before shunting procedures. It can be especially helpful to evaluate for cirrhosis, which favours a need for eventual liver transplantation.
Use of this content is subject to our disclaimer