Approach
The key to diagnosis of iatrogenic adrenal suppression is eliciting exposure to exogenous glucocorticoids or other offending agents.
Patients receiving supraphysiological doses of oral glucocorticoids for corticosteroid-responsive illnesses and having a cushingoid appearance (centripetal obesity with a round or moon facies, dorsocervical fat pads, and striae) should prompt recognition of possible hypothalamic-pituitary-adrenal (HPA) axis suppression. However, HPA axis suppression has been described after only 4 to 5 days of corticosteroid therapy and as a consequence of local glucocorticoid administration.[24][25] In patients receiving intra-articular glucocorticoid injections, recovery of the HPA axis can take 1 to 4 weeks depending on the dose and frequency of injections.[9] Although some patients appear cushingoid and some have obvious symptoms of adrenal insufficiency when the inciting corticosteroid agent is rapidly tapered or stopped, HPA axis suppression may be subtle. Not all patients appear cushingoid, and a high index of suspicion is necessary.
Patients with adrenal suppression are at risk of adrenal crisis if glucocorticoid treatment is suddenly ceased or if it is not increased during periods of increased stress (e.g., febrile illness, trauma, or surgery). Patients with a history compatible with adrenal suppression and presenting with features of adrenal crisis (i.e., hypotension, circulatory failure) should be treated urgently. Tests may be taken as baseline, but diagnostic tests should not delay treatment.[32]
History and symptoms
Patients receiving supraphysiological doses of glucocorticoids are at risk of adrenal suppression, especially if these are potent and/or administered for a prolonged period.[1][7][33] However, non-systemic corticosteroids may also cause adrenal suppression.[1] Patients may need prompting: for example, asking specifically about eye drops, nasal sprays, inhalers for breathing problems, or injections for pain.
The history should be directed to elicit the pattern of glucocorticoid use. It is important to ask about diseases for which corticosteroids are commonly prescribed, and oral or alternate routes of administration such as:
Inhaled corticosteroids for asthma or chronic obstructive pulmonary disease[11][12]
Intranasal corticosteroids for allergic rhinitis[13]
Topical corticosteroids for the treatment of dermatological conditions[14][15]
Intra-articular and epidural corticosteroids for rheumatological or pain disorders.[9][10]
Equivalent doses of hydrocortisone, prednisolone, prednisone, and dexamethasone are:[Figure caption and citation for the preceding image starts]: Dose equivalency for glucocorticoidsCreated by MC Lansang and SL Quinn [Citation ends].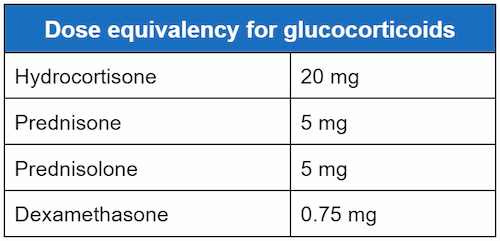
Non-prescribed sources of corticosteroids such as over-the-counter eczema treatments, skin-lightening creams, and herbal medicines may contain corticosteroids. Other sources of prescribed corticosteroids, such as enemas or rectal foam for inflammatory bowel disease, should also be sought. Additional information as to the type, dose, route of administration, and length of corticosteroid treatment should be obtained. Less commonly, patients give a history of taking medications that act on the glucocorticoid receptor, such as megestrol or medroxyprogesterone.[1][16][17] Patients should also be asked about concomitant medications that may interfere with glucocorticoid metabolism.[1] CYP3A4 is the primary pathway for the metabolism of most prescribed glucocorticoids, and so CYP3A4 inhibitors will increase exposure to glucocorticoids.[1] For example, the antiretroviral drug ritonavir is a CYP3A4 inhibitor, and is known to increase plasma concentrations of prednisone metabolites (prednisolone).[1] Other strong CYP3A4 inhibitors include antifungals such as itraconazole and ketoconazole, and cancer treatments such as ceritinib and idelalisib.[1] This list is not exhaustive, and you should consult your local drug formulary. Rarely, patients have a history of removal of a pituitary adenoma or carcinoma that caused Cushing's syndrome.[19][20]
Symptoms of adrenal insufficiency may be present, especially when the inciting agent is rapidly tapered or stopped. In patients with glucocorticoid-induced adrenal suppression, interaction with medications that induce CYP3A4 will decrease synthetic glucocorticoid levels and may also cause symptoms of adrenal insufficiency. Examples of agents that induce CYP3A4 include carbamazepine, phenobarbital, phenytoin, primidone, rifampicin, and nafcillin.[1] This list is not exhaustive, and you should consult your local drug formulary. Symptoms of adrenal insufficiency are generally vague. They include fatigue, anorexia, nausea and/or vomiting, and weight loss.[32] Dizziness, orthostatic symptoms, myalgias, and arthralgias may also be present. Abdominal pain can be mild or severe enough to lead to a misdiagnosis of acute abdomen.
Patients may report symptoms consistent with Cushing's syndrome, including central nervous system symptoms such as depression, agitation, or sleep disorders.[34] There may be a prior history of weight gain and increased appetite, round face, dorsocervical fat pads, and easy bruising. Other medical disorders such as hypertension and diabetes may have become more difficult to control and less responsive to medication.
Adrenal suppression in the paediatric population can present as an adrenal crisis in times of stress, but is more likely to be subtle. The non-specific symptoms of adrenal suppression in children are similar to those seen in adults; however, children may also exhibit growth failure and hypoglycaemia.[2]
Physical examination
Patients at risk of adrenal suppression who have suddenly ceased their glucocorticoid medication, or not increased the dose during periods of increased stress, can present with an acute adrenal crisis with collapse due to hypovolemic shock, hypotension, postural dizziness, and tachycardia.[32]
In less acute cases, patients may or may not appear cushingoid and hypertension may be present. Proximal muscle weakness may also be present. The complexion may be plethoric with pigmented striae; thin, easily bruised skin; and acne. Features associated with elevations of adrenocorticotropic hormone (ACTH) such as mucosal or cutaneous hyperpigmentation will be absent. Also likely to be absent are other stigmata of autoimmune disorders associated with autoimmune adrenal insufficiency, such as vitiligo. These features help distinguish iatrogenic from endogenously induced adrenal suppression.
Tests
In cases of acute adrenal crisis, diagnostic tests should not delay treatment.[32] Investigations will include close monitoring of blood pressure, fluid balance, and baseline bloods (full blood count, electrolytes, urea, creatinine, thyroid function [hyperthyroidism can trigger adrenal crisis], cortisol, and ACTH level). If the patient is haemodynamically stable, consider a short ACTH stimulation test.[32]
Although not diagnostic, incidental laboratory findings such as hypo- or hyperglycaemia, hypokalaemia, hypomagnesaemia, and a contraction alkalosis may raise suspicion of glucocorticoid use. Electrolyte abnormalities consistent with mineralocorticoid deficiency such as hyperkalaemia are absent because the renin-angiotensin-aldosterone system remains intact.
As long as supraphysiological corticosteroids continue, evaluation of adrenal function is not helpful. Patients with a history of glucocorticoid use who have had discontinuation or a recent reduction in corticosteroid dose to physiological or sub-physiological levels, along with symptoms of adrenal insufficiency, should be tested for adrenal suppression.
If corticosteroids have been discontinued, an early morning random cortisol is the simplest diagnostic test, but results are usually inconclusive, necessitating one of the more cumbersome but more reliable stimulation tests for confirmation. Because ACTH has been suppressed by the exogenous corticosteroid, serum cortisol is expected to be low. Values below 110 nanomol/L (4 micrograms/dL) are consistent with HPA axis suppression. Values between 110 and 469 nanomol/L (4 and 17 micrograms/dL) are inconclusive.[35] Indeterminate values require confirmation with one of the stimulation tests to safely discontinue corticosteroid therapy. Random serum cortisol levels, or ACTH levels, are not recommended as reliable indicators of adrenal status. In lieu of completing one of the stimulation tests, many physicians prefer to proceed with a corticosteroid tapering programme. When the morning serum cortisol value is >497 nanomol/L (18 micrograms/dL), corticosteroids can be safely discontinued.
Midnight salivary cortisol has been recognised as an excellent diagnostic tool for identifying Cushing's syndrome but there has been increased interest in morning salivary cortisols as a means of screening for adrenal insufficiency. Studies have found that morning serum cortisol levels are equivalent to salivary cortisol levels at differentiating adrenal insufficiency from normal adrenal function.[36][37] Salivary cortisols may be especially useful for patients with hepatic disease who have hypoalbuminaemia and cirrhosis where salivary cortisol levels correlate better with adrenal function and plasma free cortisol than do total plasma cortisols.[38][39] Salivary cortisol may also be useful as a surrogate for plasma free cortisol during the ACTH stimulation test for patients with other forms of increased or decreased plasma binding protein levels.[40] In intensive care unit (ICU) patients, salivary and serum free cortisols have a close correlation, provided adequate saliva samples are obtained; however, inadequate sampling and contamination was found to be frequent problem.[41][42] Although it is likely salivary cortisols will become more widely used in the future it is best to use an increase in total serum cortisol of <9 micrograms/dL after ACTH 250 micrograms or a random total cortisol of <10 micrograms/dL to confirm adrenal insufficiency in the ICU patient.[43]
ACTH stimulation tests are the preferred methods of detecting adrenal suppression. The conventional ACTH and the low-dose ACTH stimulation tests use ACTH (synthetic ACTH such as cosyntropin injections or infusions) to measure adrenal cortisol output and are generally reliable. The 250 microgram dose is the most commonly used, and the test can be performed even in the office setting.[44] Using 1 or 10 micrograms of ACTH compared with 250 micrograms has been suggested as more sensitive in identifying adrenal hypofunction, but these preparations require dilutions and intravenous infusions rather than the simpler intramuscular and intravenous injections by which the 250 microgram dose is administered.[45][46][47][48][49] Historically, cut-off 30-minute values for the 1-microgram test have been acknowledged as <18 to 20 microgram/dL in non-stressed patients and <25 microgram/dL or an increase of <9 microgram/dL from baseline in critically ill patients.[50] However, newer monoclonal assays or liquid chromatography mass spectrometry methods to measure serum cortisol concentrations are associated with reduced cross reactivity with other glucocorticoids, and therefore a lower 30- or 60- peak serum cortisol cut-off of <15 microgram/dL has been proposed.[1][51] A meta-analysis comparing the 1 mcg ACTH stimulation test versus the 250 mcg dose showed low sensitivity and high specificity of each for diagnosing secondary adrenal insufficiency, with similar diagnostic accuracy of the two doses.[52] The ACTH stimulation test may be unreliable in patients with recent onset of HPA axis suppression where the adrenal glands have not had sufficient time to atrophy. If patients are taking hydrocortisone or prednisolone, it is recommended to withhold the treatment for 24 hours before the test in order to avoid false positives. Other corticosteroid preparations, such as dexamethasone, do not cross-react with the cortisol assay used for the ACTH stimulation test.
The insulin tolerance test (ITT) and the overnight metyrapone test, although somewhat cumbersome, evaluate the entire HPA axis and are capable of assessing partial adrenal suppression.[44] ITT can be used if there is concomitant need to determine whether the patient also has growth hormone deficiency, for which the ITT is also a diagnostic test. Both ITT and metyrapone tests can be used if it is pertinent to know whether the patient has secondary adrenal insufficiency due to a recent pituitary insult (e.g., pituitary surgery). In this situation, the adrenal glands can still mount a normal response to the ACTH stimulation test up to 2 to 3 weeks after the pituitary insult because of adrenal reserve. However, the ITT may uncover that the pituitary gland is not capable of responding to stress. Because the ITT relies on production of symptomatic hypoglycaemia to stimulate cortisol release, it evaluates the entire HPA axis but has the associated risk of hypoglycaemic seizures and, therefore, must be performed under close observation. It is not recommended for frail or older patients with cardiovascular disease or seizures. The metyrapone test also evaluates the entire HPA axis but is associated with the theoretical, although unlikely, potential to precipitate acute adrenal insufficiency.
In patients who have a vague recollection of being given local glucocorticoid preparations (such as epidural or intra-articular) suspected to be causing adrenal suppression, screening of urine for synthetic corticosteroids will confirm systemic absorption of exogenous glucocorticoids but is not diagnostic of adrenal suppression.[Figure caption and citation for the preceding image starts]: Adrenal suppression tests tableCreated by MC Lansang and SL Quinn [Citation ends].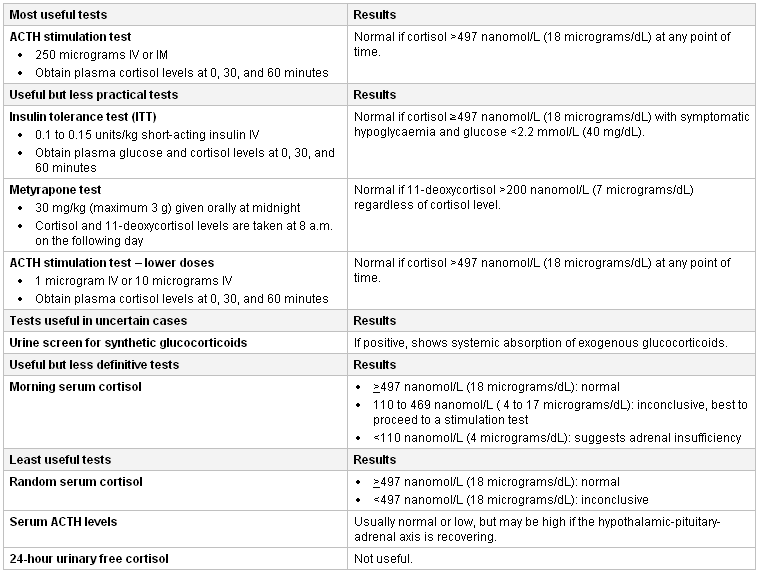
Use of this content is subject to our disclaimer