Approach
NSVT is defined as an ectopic ventricular rhythm on ECG consisting of 3 or more consecutive, wide (duration 120 milliseconds or greater) QRS complexes with a heart rate greater than 100 bpm, spontaneously resolving in less than 30 seconds.[1][2] Once the presence of an NSVT is established, the main goals are to identify any existing cardiac pathology and to risk stratify patients with known cardiac disease for appropriate management and therapy.
[Figure caption and citation for the preceding image starts]: Evaluation of non-sustained wide QRS tachycardiaCreated by BMJ Knowledge Centre. [Citation ends].
NSVT is usually asymptomatic on presentation, and symptoms such as palpitations and syncope overlap greatly with other pathologies and tachyarrhythmias. Therefore, further investigation is required to identify the specific aetiology of NSVT.[2] It is appropriate to begin with a complete history and physical examination followed by ECG. Laboratory studies and echocardiography may also be useful in the initial workup of the patient. More expensive and invasive testing such as magnetic resonance imaging (MRI) and cardiac catheterisation should be ordered in the appropriate clinical setting after initial evaluation has been completed.
History
NSVT has been identified in a wide spectrum of patients, from those with no identifiable cardiac condition to patients with ischaemic and non-ischaemic cardiac disease. Therefore, it is important to assess the patient's risk factors in light of NSVT that would prompt further testing and/or therapy.
History that places patients at higher risk:
Non-ischaemic cardiomyopathy with left ventricular ejection fraction (LVEF) <35% and New York Heart Association class II or III symptoms
Ischaemic cardiomyopathy with LVEF <35%
Ischaemic cardiomyopathy with LVEF <40% with NSVT and inducible ventricular tachycardia (VT) during electrophysiological testing
Hypertrophic cardiomyopathy (HCM) with personal history of cardiac arrest or sustained VT, first-degree relative with sudden death, unexplained exertional or recurrent syncope, left ventricular septum thickness greater than 30 mm, or frequent or prolonged NSVT
Congenital arrhythmia syndromes such as long QT and Brugada's syndromes.[31]
Family history of structural heart disease, electrical disease, and cardiomyopathy should be assessed.[2] In addition, the patient should be questioned regarding the use of any potentially arrhythmogenic medication (e.g., macrolide antibiotics, chlorpromazine, haloperidol, digoxin, flecainide, sotalol, and dofetilide). Family history of cardiac disease or sudden cardiac death may be of significance. The physician should also enquire about the presence of any current mental or physical stress that may have triggered the arrhythmia. Patients may complain of syncope as a result of hypotension due to the NSVT or an underlying cardiac condition. Dizziness, lightheadedness, and pre-syncope may occur if cerebral blood flow is compromised. Syncope suspected to be of cardiac origin is a high-risk symptom that may require rapid hospital admission for further investigation.[2]
Physical examination
This should focus on possible signs for occult or worsening cardiac disease in patients with no known cardiac condition or patients with known cardiac disease, respectively.
Palpation of peripheral pulses provides information on heart rate, identifying a current tachycardia and in combination with peripheral BP monitoring can suggest the status of the patient's cardiac output. The presence of rales on chest examination or the presence of peripheral oedema may be a sign of elevated left or right atrial pressures associated with cardiac dysfunction. Similarly, palpation of the heart for left or right ventricular heaves and examination of the neck for elevated jugular venous pressure should be performed. Careful auscultation for pathological cardiac murmurs or abnormal heart sounds (S3 or S4) may assist in prompting further workup for ischaemic and non-ischaemic cardiac disease.
ECG
NSVT is diagnosed based on the following specific ECG findings: a ventricular rhythm with wide (120 milliseconds or greater) QRS complex; and a rate faster than 100 bpm lasting for at least 3 beats that spontaneously resolves in less than 30 seconds. [Figure caption and citation for the preceding image starts]: Non-sustained ventricular tachycardiaFrom the collection of Dr F. Kusumoto; used with permission [Citation ends].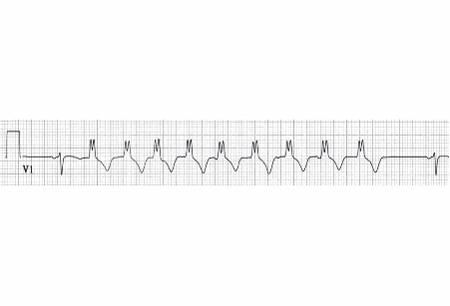
A 24-hour ambulatory ECG may be necessary to detect the NSVT, determine the extent of the arrhythmia burden and/or analyse the QT interval (can be identified using a standard 12-lead ECG, although at times temporally dynamic changes in the QT interval require more prolonged monitoring periods such as a 24-hour ambulatory monitor). Using at least 3 leads on the Holter may help estimate whether the NSVT/premature ventricular contractions (PVCs) are uni- or multi-focal, and show their site of origin.[2] More recently, consumer wearable devices that may identify arrhythmias, including NSVT, have been used by patients. While the quality of the recordings from these devices has improved dramatically, particularly in identifying atrial fibrillation and identification and assessment of burden of ventricular arrhythmias, medical grade ECG recording devices provide more detailed and clinically actionable information.[32]
It is important to differentiate between a wide complex tachycardia originating from the ventricles and one that is supraventricular, as the medical management differs.
Evidence supporting the diagnosis of a VT includes:
Baseline ECG tachycardia with morphology similar to that of PVCs
Initiation of the tachycardia with a PVC making NSVT more likely.[Figure caption and citation for the preceding image starts]: Initiation of wide QRS rhythm is not preceded by a P-wave, suggesting non-sustained ventricular tachycardiaKusumoto FM. ECG interpretation. In: Pathophysiology to clinical application. New York, NY: Springer; 2009; used with permission [Citation ends].
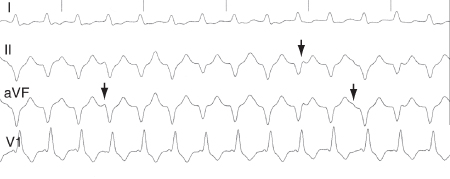
AV dissociation during tachycardia with dissociated P waves or capture and fusion beats[Figure caption and citation for the preceding image starts]: ECG showing AV dissociation. Deflections due to P waves (arrows) are not associated with QRS complexesKusumoto FM. ECG interpretation. In: Pathophysiology to clinical application. New York, NY: Springer; 2009; used with permission [Citation ends].
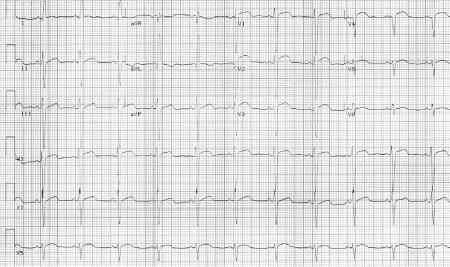
QRS duration greater than 140 milliseconds with right bundle branch block (RBBB) morphology, or QRS duration greater than 160 milliseconds with left bundle branch block (LBBB) morphology; however, does not apply to patients on antiarrhythmic drugs.[33]
A right superior axis or LBBB morphology with any right axis[1]
QRS complexes broadly positive in the inferior leads (1, 3, and aVF), in which case NSVT associated with an idiopathic site from the ventricular outflow tracts should be suspected. If the site is within the right ventricular outflow tract, the QRS complex will have LBBB morphology (negative in V1), and if the site is within the left ventricular outflow tract, it will have RBBB morphology (positive QRS in V1). This is important to identify from a clinical standpoint, as these entities are not associated with sudden cardiac death and are often treated with ablation.
Baseline ECG should also be examined for evidence of known risk factors for NSVT including:
Long QT syndrome: QT interval prolongation[Figure caption and citation for the preceding image starts]: ECG from a patient with long QT syndromeKusumoto FM. ECG interpretation. In: Pathophysiology to clinical application. New York, NY: Springer; 2009; used with permission [Citation ends].

Brugada's syndrome: J point elevation and downwards-sloping ST-segment elevation in the right pre-cordial leads[Figure caption and citation for the preceding image starts]: ECG from a patient with Brugada's syndrome, showing terminal positive R-wave and ST-segment elevation in lead V1Kusumoto FM. ECG interpretation. In: Pathophysiology to clinical application. New York, NY: Springer; 2009; used with permission [Citation ends].

Arrhythmogenic right ventricular cardiomyopathy: interventricular conduction delays (a discrete deflection after the QRS complex called an epsilon wave in lead V1 is specific for this disease)[Figure caption and citation for the preceding image starts]: ECG from a patient with arrhythmogenic right ventricular cardiomyopathyKusumoto FM. ECG interpretation. In: Pathophysiology to clinical application. New York, NY: Springer; 2009; used with permission [Citation ends].

Electrolyte disorders: prolonged QT interval
Presence of Q waves, ST-segment abnormalities, or other signs of coronary artery disease (CAD)[Figure caption and citation for the preceding image starts]: ECG showing an abnormal Q wave in V4 and ST elevation in V1-V6 in a patient with a large anterior wall myocardial infarctionKusumoto FM. ECG interpretation. In: Pathophysiology to clinical application. New York, NY: Springer; 2009; used with permission [Citation ends].
LBBB: may be a sign of structural heart disease, including Chagas' disease or dilated cardiomyopathy
T-wave inversion: although non-specific, may be an important marker of underlying structural cardiac disease.
The morphology of the NSVT can give clues to its cause, with typical monomorphic VT suggesting an idiopathic cause with good outcome.[2] Some morphologies are particularly concerning. For example, longer bursts of polymorphic VT, particularly those with rapid rates (shorter duration [cycle length] between QRS complexes), or short-coupled PVCs that initiate NS polymorphic VT or monomorphic NSVT with short cycle length can indicate patients at higher risk of sudden cardiac death. These patients may need to be admitted for further assessment.[2] The evidence for the prognostic value of the NSVT duration (e.g., 6 beats vs. 25 beats) has been mixed, however, as a general rule longer episodes raise more concern than shorter episodes (<3-5 beats).
Electrolytes and cardiac bio-markers
Electrolyte abnormalities, especially hypokalaemia, hyperkalaemia, and hypo-magnesaemia, can often trigger and/or contribute to NSVT, and therefore any blood electrolyte abnormality must be identified.[2] Clinical suspicion for ischaemia should prompt testing of myocardial bio-marker assays (CK-MB and troponin I), as they provide useful confirmatory information regarding myocardial infarction (MI).
Echocardiogram, stress test, cardiac catheterisation, or MRI
Investigation for underlying cardiac disease is facilitated by several diagnostic tests.
Echocardiography is a safe and inexpensive tool to evaluate for structural cardiac abnormalities, such as valvular heart disease, which in advanced stages can cause NSVT and is treatable. Echocardiography is also useful to quantify systolic function and detect the presence of an underlying cardiomyopathy.[2]
Stress testing and/or cardiac catheterisation is useful in establishing the presence of CAD in patients who have risk factors for CAD but may be asymptomatic. The patient's pre-test probability of CAD should be scored and used to guide the choice of investigations. An exercise stress test can identify exercise-induced arrhythmias, and, if present, may indicate underlying structural heart disease. Patients may need to be advised to avoid exercise until they have undergone further investigation and treatment.[2] Often, stress testing with imaging (e.g., nuclear or echocardiography) is required for more definitive identification of the underlying ischaemia.
Cardiac MRI with gadolinium may be useful for identifying myocardial scarring associated with CAD, dilated idiopathic cardiomyopathy, and HCM. It also confirms the presence of fibrofatty infiltrates in the ventricle, and measures right ventricular dysfunction in patients with suspected arrhythmogenic right ventricular cardiomyopathy. Guidelines recommend considering a cardiac MRI in patients with newly documented NSVT and suspected structural heart disease (other than CAD) after initial evaluation.[2]
Electrophysiological testing
Consultation with an electrophysiologist is essential to determining which patients would benefit from an invasive electrophysiological (EP) test, implantable cardioverter defibrillator placement, and medical treatment optimisation.[1] EP testing can be useful for evaluation (and treatment) in a wide range of patients after MI, including those with inducible VT and ejection fraction (EF) less than 40%, and those with EF less than 30% to 35% without inducible VT, especially if QRS is prolonged.[31][34] In patients with symptomatic NSVT (dizziness, syncope) that arises from the outflow tract, EP testing and ablation is an important diagnostic and therapeutic tool.
Genetic screening
Genetic screening is available for common mutations associated with long QT syndrome, Brugada's syndrome, and catecholaminergic polymorphic VT, as well as HCM and dilated cardiomyopathy. Consultation with a cardiologist or a medical geneticist may be helpful in determining which patients would benefit the most from genetic screening. Cascade genetic testing of family members may be warranted.[2][23][35][36][37]
Use of this content is subject to our disclaimer