Approach
Failure to identify obesity in children, the primary risk factor for type 2 diabetes mellitus (T2DM), results in missed opportunities for risk assessment, testing for T2DM and other obesity-related comorbidities, and counselling about lifestyle modifications.
History
The majority of children with T2DM have overweight. Children from high-risk racial/ethnic backgrounds, including black and Indo-Asian, are more commonly affected.
Children with T2DM often have mild or absent polyuria, nocturia, and polydipsia at presentation. They also only rarely present with weight loss. They may complain of fatigue, blurred vision, or yeast infections in intertriginous areas. Patients with severe obesity should be questioned about clinical signs of obstructive sleep apnoea, including daytime fatigue, snoring, and nocturnal pauses in breathing >5 seconds. Although diabetic ketoacidosis is less common in T2DM than in type 1 diabetes mellitus (T1DM), beta-cell dysfunction due to insulinopenia from glucose toxicity results in ketosis in up to one third and ketoacidosis in 15% of children with T2DM at presentation. There may be a history of recurrent skin or urinary tract infections.
A positive history of T2DM in a first- or second-degree relative may be present. Additionally, history of maternal obesity and gestational diabetes should be obtained, if present. The child may have been born with a low birth weight and may have a history of rapid catch-up in infancy. Obtaining a history of feeding practices in infancy is recommended, as breastfeeding reduces the odds ratio for childhood obesity by approximately 20% compared with formula feeding.[41] Girls can be questioned about their menstrual history to evaluate for polycystic ovarian syndrome.
Screening for T2DM is recommended for any Australian Aboriginal or Torres Strait Islander person aged >10 years (or past the onset of puberty) with overweight or obesity, who has a positive family history of diabetes, has signs of insulin resistance, has dyslipidaemia, has received psychotropic therapy, or has been exposed to diabetes in utero.[54]
Physical examination
General practitioners must calculate and plot body mass index (BMI) at each visit to identify children who have, or are at risk for developing, obesity (i.e., crossing percentiles on BMI chart). Waist circumference should be plotted on the appropriate chart for age, gender, and ethnicity, and should be used as an indicator of visceral distribution of fat. The majority of children with T2DM will have a BMI and waist circumference >85th percentile.
The patient can be examined for the presence of acanthosis nigricans, seen in 90% to 95% of patients with T2DM. This is a cutaneous manifestation of insulin resistance characterised by velvety, hyper-pigmented skin, most often in the intertriginous areas such as in the axilla or in the neck. However, acanthosis nigricans is not specific for T2DM and can also be seen in children with obesity and T1DM. Other skin changes may include cellulitis or abscesses. The patient should be examined for yeast infections, the most frequent sites being the vaginal and penile areas and between skin folds.
Pubertal status needs to be carefully assessed as a pre-pubertal status in patients with T2DM is rare and should raise suspicion of T1DM.[Figure caption and citation for the preceding image starts]: Acanthosis nigricansFrom the collection of Dr Jennifer Miller [Citation ends].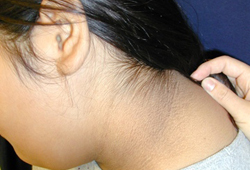 [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in a child with obesityFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in a child with obesityFrom the collection of Dr Jennifer Miller [Citation ends]. [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the folds of the neckFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the folds of the neckFrom the collection of Dr Jennifer Miller [Citation ends].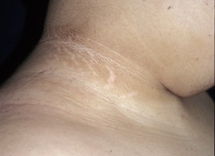 [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the axillaFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the axillaFrom the collection of Dr Jennifer Miller [Citation ends].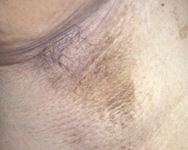
Diagnostic tests
One of four tests can be used to confirm hyperglycaemia and make the diagnosis:[1]
Fasting plasma glucose ≥7.0 mmol/L (≥126 mg/dL)
Random plasma glucose of ≥11.1 mmol/L (≥200 mg/dL) with classic symptoms of hyperglycaemia or hyperglycaemic crisis
Plasma glucose ≥11.1 mmol/L (≥200 mg/dL) 2 hours after 75 oral glucose
Haemoglobin A1c (HbA1c) of ≥48 mmol/mol (6.5%).
In the absence of unequivocal hyperglycaemia, the diagnosis requires two abnormal test results from the same sample, or in two separate test samples.[1]
Additional investigations
It is recommended that a baseline HbA1c is measured in all patients at the time of diagnosis. It indicates the average blood glucose over the previous 3 months and is a useful test in monitoring of diabetic control.[1]
C-peptide is produced in equal amounts to insulin and is the best measure of endogenous insulin secretion in patients with diabetes. There is no role for routine testing for C-peptide for diagnosis of diabetes, but measuring C-peptide may be useful in differentiating T1DM from T2DM.[55] The best evidenced C-peptide test is the glucagon stimulation test (GST), but non-fasting 'random' blood C-peptide has been shown to correlate with fasting C-peptide and post-GST samples in individuals with well-defined T1DM or T2DM.[56] Development of absolute insulin deficiency is a key feature of T1DM, which results in low (<0.2 nanomol/L) or undetectable levels of plasma C-peptide.[55] A GST or non-fasting 'random' blood C-peptide level >1 nanomol/L suggests T2DM.[55] C-peptide results must be interpreted in the clinical context of disease duration, comorbidities, and family history.[56]
Although C-peptide can be helpful in evaluating the endogenous production of insulin, both T1DM and T2DM can be associated with insulinopenia, and endogenous insulin production can be detected in some individuals with T1DM for prolonged periods of time after diagnosis. Testing for autoimmunity is therefore more helpful in identifying immune-mediated diabetes, the most prevalent form of T1DM.
Autoantibodies to insulin, glutamic acid decarboxylase, islet cells, islet antigens, and zinc transporter 8 should be measured in all patients to exclude a diagnosis of T1DM.[1] Results of pancreatic autoantibody testing may not always be available at the initiation of treatment. Treatment will need to be adjusted if patients are positive for pancreatic autoantibodies and a diagnosis of type 1 diabetes is confirmed. For more information, see Type 1 diabetes mellitus.
It is also important to consider monogenic diabetes in the differential for type of diabetes, because this accounts for up to 4% of paediatric diabetes cases.[57] The two main forms of monogenic diabetes are maturity-onset diabetes of the young (MODY) and neonatal diabetes.[1] Genetic testing for MODY is required in those without the typical signs of T1DM or T2DM (e.g., no obesity, negative autoantibodies) and who have a family history of diabetes in successive generations.[1] Genetic testing for neonatal diabetes is necessary if an individual is diagnosed with diabetes in the first 6 months of life.[1]
It is recommended that blood pressure measurement, a fasting lipid profile, albuminuria assessment, liver function testing, and dilated fundascopy eye examination be performed at the time of diagnosis.[1]
Hypertension: blood pressure should be checked at each visit using an appropriate-sized cuff while the child is seated at rest.[1][58] The levels should be compared with age-, sex-, and height-appropriate standards. Hypertension is frequently present at the time of diagnosis. Ambulatory blood pressure monitoring should be strongly considered if blood pressure is high (blood pressure ≥90th percentile for age, sex, and height or, in adolescents aged ≥13 years, ≥120/80 mmHg) on three separate measurements.[1]
Dyslipidaemia: a fasting lipid profile, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) for metabolic dysfunction-associated steatotic liver disease (formerly called non-alcoholic fatty liver disease), should be checked at diagnosis, after diagnosis, and annually thereafter.
Diabetic retinopathy: dilated fundoscopy eye examination should be performed at diagnosis, and annually thereafter in most patients. Examination every 2 years may be appropriate if glycaemic targets are met and the previous eye examination is normal.
Diabetic nephropathy: urine albumin-to-creatinine ratio and estimated glomerular filtration rate should be evaluated at diagnosis and annually thereafter. Referral to a specialist is warranted in the presence of worsening albumin-to-creatinine ratio or a decrease in eGFR.
Diabetic neuropathy: inspect the feet, assess foot pulses, perform pinprick and 10 -g monofilament sensation tests, test vibration sensation using a 128 Hz tuning fork, and test ankle reflexes at diagnosis and annually thereafter.
Obstructive sleep apnoea: screening for symptoms should be carried out at each visit, with referral to a paediatric sleep specialist for evaluation and a polysomnogram if screening is positive.[1]
Polycystic ovary syndrome: female adolescents with T2DM should be evaluated for polycystic ovary syndrome symptoms at diagnosis and at subsequent reviews, with laboratory studies where indicated.[1]
Psycho-social assessment is also important; at diagnosis and during routine follow-up care, screen for psycho-social issues and family stressors which might impact negatively on diabetes management, such as diabetes distress, depressive symptoms, disordered eating, family factors, and behavioural health concerns. Refer to a trained mental health professional (preferably one experienced in childhood diabetes) as required for further assessment and treatment.[1] Specific screening for psycho-social and diabetes related distress is recommended from around the age of 7-8 years.[1]
Complications should continue to be monitored and treated.[1] For more detail on ongoing monitoring, see Monitoring.
Use of this content is subject to our disclaimer