Aetiology
Vesicular-bullous disorders may have an autoimmune, infectious, genetic, inflammatory, extrinsic, mechanical, or metabolic aetiology.[4][5]
Infectious causes
Bacterial infections
Impetigo is a common, highly contagious, superficial skin infection that primarily affects children. The condition can present in both non-bullous and bullous forms.
Staphylococcal scalded skin syndrome or Ritter's disease/pemphigus neonatorum is an uncommon manifestation of a staphylococcal infection.
The cutaneous manifestations of syphilis can include blisters, especially when occurring in the neonatal period.[6] Syphilis is a communicable disease caused by Treponema pallidum.[Figure caption and citation for the preceding image starts]: Impetigo of arm presenting as an erosionFrom the collection of Michael Freeman [Citation ends].
 [Figure caption and citation for the preceding image starts]: Florid bullous impetigoFrom the collection of Michael Freeman [Citation ends].
[Figure caption and citation for the preceding image starts]: Florid bullous impetigoFrom the collection of Michael Freeman [Citation ends].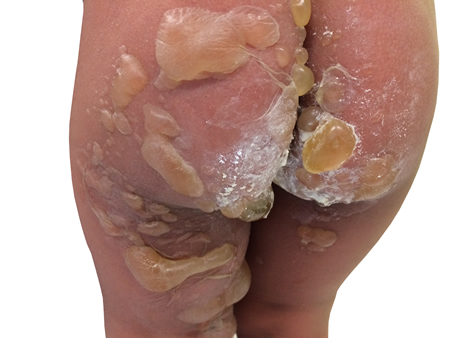
Viral infections
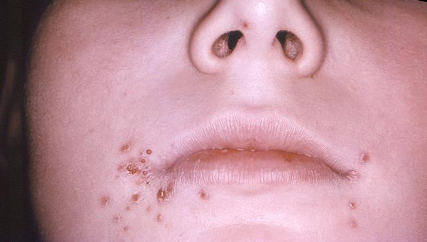
Herpes simplex virus (HSV) can cause primary, latent, and recurrent infections. HSV-1 is more frequently associated with orolabial herpes. HSV-2 is the cause of most genital herpes infections (70% to 90%), although the incidence of HSV-1 infection is increasing (from 10% to 30%) in this context.[7] HSV is associated with erythema multiforme and is thought to trigger this condition.[Figure caption and citation for the preceding image starts]: Lesions caused by herpes simplex virus Type-1 (HSV-1)CDC/Robert E. Sumpter [Citation ends].
 [Figure caption and citation for the preceding image starts]: HSV-penile vesicular lesionFrom the collection of Christine Johnston, MD and Anna Wald, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: HSV-penile vesicular lesionFrom the collection of Christine Johnston, MD and Anna Wald, MD [Citation ends].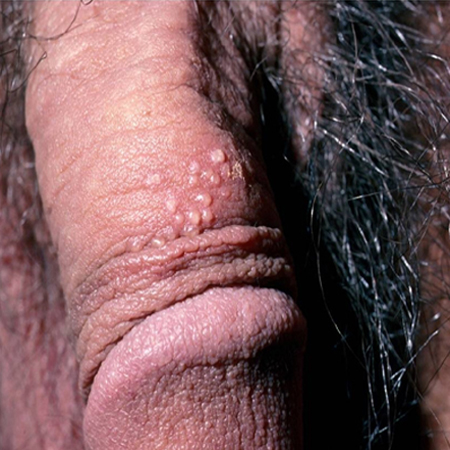 [Figure caption and citation for the preceding image starts]: HSV-labial ulcerationFrom the collection of Christine Johnston, MD and Anna Wald, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: HSV-labial ulcerationFrom the collection of Christine Johnston, MD and Anna Wald, MD [Citation ends]. [Figure caption and citation for the preceding image starts]: Target lesions on the face and mucosal erosions with crusting due to HSV-1 recurrenceFrom the personal collection of Nanette Silverberg, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Target lesions on the face and mucosal erosions with crusting due to HSV-1 recurrenceFrom the personal collection of Nanette Silverberg, MD [Citation ends].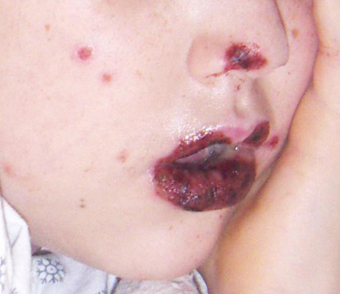
Herpes zoster virus (HZV) infection results from re-activation of latent varicella infection and develops in approximately 20% of healthy adults and 50% of immunocompromised people.[7] HZV can occur any time after the primary varicella infection.
Varicella zoster virus infection, commonly known as chickenpox, predominantly affects children. More than 90% of cases occur in children <10 years of age.[1] Transmission occurs by the respiratory route or direct contact. The incubation period is 10-21 days. A typical patient is infectious approximately 4 days before and 5 days after the onset of lesions.[Figure caption and citation for the preceding image starts]: Varicella zoster virus (VZV)Courtesy of Daniel Eisen, MD [Citation ends].
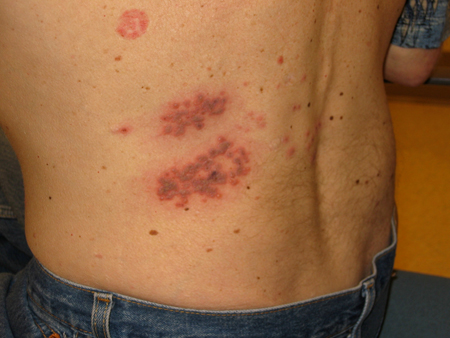 [Figure caption and citation for the preceding image starts]: Varicella zoster virus (VZV)Courtesy of Daniel Eisen, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Varicella zoster virus (VZV)Courtesy of Daniel Eisen, MD [Citation ends].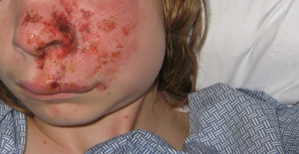 [Figure caption and citation for the preceding image starts]: Varicella lesion on hard palate of a young patientCDC Public Health Image Library [Citation ends].
[Figure caption and citation for the preceding image starts]: Varicella lesion on hard palate of a young patientCDC Public Health Image Library [Citation ends].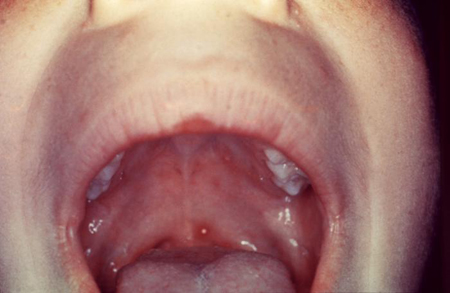 [Figure caption and citation for the preceding image starts]: Typical vesicular rash of primary varicella; note that lesions are in different stagesCDC Public Health Image Library [Citation ends].
[Figure caption and citation for the preceding image starts]: Typical vesicular rash of primary varicella; note that lesions are in different stagesCDC Public Health Image Library [Citation ends].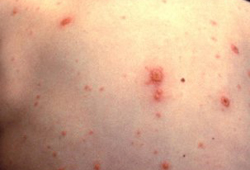
Hand-foot-and-mouth disease is often due to infection with coxsackievirus. Coxsackievirus A16 infection is the most common cause, but A4, A5, A6, A7, A9, and A10 infections also occur.[8]
Mpox is caused by a double-stranded DNA virus.[9] The disease is endemic in West and Central Africa; however, a global outbreak was identified in May 2022.[10] In this ongoing global clade II mpox outbreak, chains of transmission have been reported in countries without known epidemiological links to West and Central Africa (for the first time). There are also currently ongoing clade I mpox outbreaks in Africa, with a small number of travel-related cases reported in countries outside of Africa. The World Health Organization declared the current mpox outbreaks in Africa a public health emergency of international concern (PHEIC) in August 2024.[11] Incubation period is typically 6-13 days (range 1-21 days).[12][13] Mpox typically presents with characteristic rash that progresses in sequential stages from macules, to papules, vesicles, and pustules.[14][15] However, the clinical presentation has been atypical in recent outbreaks. Human‐to‐human transmission can occur through direct contact with skin lesions or infected skin, respiratory droplets and fomites, or through the transplacental route.[9] Sexual transmission within sexual networks, mainly men who have sex with men and commercial sex workers, has been a key driver of both the global clade II and clade I mpox outbreaks.
Fungal infections
Dermatophytes are fungal organisms that require keratin for growth. These fungi can cause superficial infections of the hair, skin, nails, and bullae. Dermatophytes are spread by direct contact with other people, animals, soil, and fomites. When infection involves the skin, it is commonly caused by the 'ringworm' fungi (Microsporum, Trichophyton, and Epidermophyton). Cutaneous ringworm on non-scalp skin presents as slowly enlarging, scaly, erythematous annular lesions with central clearing. Of most relevance is dermatophyte infection with Trichophyton mentagrophytes, which can cause bullous tinea pedis. This form of dermatophyte (tinea) infection presents with multi-locular bullae involving the thin skin of the plantar arch and along the sides of feet and heel.
Parasitic infections
Scabies is a highly contagious infestation caused by the ectoparasite Sarcoptes scabiei, which lives within the epidermis of the skin.[16] Transmission is primarily through direct contact with an infested person or persons. Epiluminescence microscopy or dermoscopy can be useful to visualise the mites or eggs in vivo.[17][Figure caption and citation for the preceding image starts]: Scabie mite under 10 x powerFrom the collection of Laura Ferris, MD, PhD [Citation ends].
 [Figure caption and citation for the preceding image starts]: Characteristic linear burrows in skinFrom the collection of Laura Ferris, MD, PhD [Citation ends].
[Figure caption and citation for the preceding image starts]: Characteristic linear burrows in skinFrom the collection of Laura Ferris, MD, PhD [Citation ends].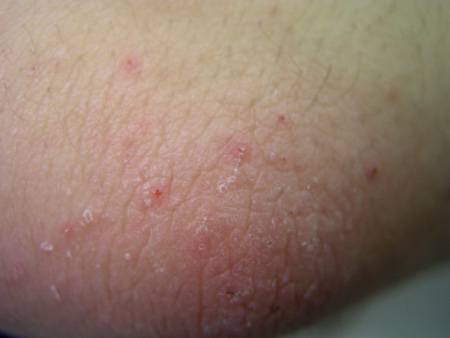
Mechanical causes
Epidermolysis bullosa (EB) is a rare heterogeneous group of genodermatoses, characterised by skin and mucous membrane fragility, and by the formation of blisters in response to minor physical injury.[18] The inheritance of these conditions can be either autosomal dominant or autosomal recessive. There are 3 main subtypes:[18][19]
EB simplex (EBS: intraepidermal blister formation)
Junctional EB (JEB: blister formation in lamina lucida or central basement membrane zone)
Dystrophic EB (DEB: sublamina densa blister formation). [Figure caption and citation for the preceding image starts]: Recessive dystrophic epidermolysis bullosa (DEB)Courtesy of Daniel Eisen, MD [Citation ends].
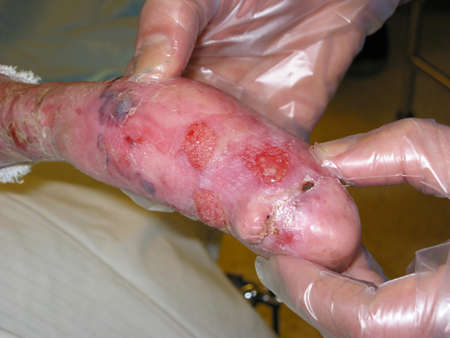 [Figure caption and citation for the preceding image starts]: Recessive dystrophic epidermolysis bullosa (DEB)Courtesy of Daniel Eisen, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Recessive dystrophic epidermolysis bullosa (DEB)Courtesy of Daniel Eisen, MD [Citation ends].
Infection, sepsis, and death may complicate generalised blistering caused by any subtype of EB, especially in early childhood. However, these complications are more common in JEB and DEB, and rarer in EBS. The risk of death is increased in severe forms of EB, specifically patients with JEB, who have the highest mortality during infancy. For those patients with recessive DEB who survive to adulthood, the most common cause of death is metastatic squamous cell carcinoma. These patients need to be followed closely, as the risk of squamous cell carcinoma is increased due to poor healing of chronic wounds. In contrast, dominantly inherited EBS and DEB, and milder forms of JEB, often have a normal life expectancy.
Other mechanical processes:
Friction blisters occur at sites of combined pressure and friction, and create localised discomfort. Hot and moist environments may enhance the development of blisters, the extent of which often depends on the extent and anatomical site of the trauma[1]
Coma bullae with sweat gland necrosis are observed in patients who are in a comatose state[20]
Miliaria is the term used for the retention of sweat as a result of eccrine sweat duct occlusion. It is commonly seen in hot, humid climates in people with clothing preventing dissipation of heat and moisture or in acute febrile illness in hospitalised patients.[Figure caption and citation for the preceding image starts]: Miliaria crystallina in hospitalised febrile patientFrom the collection of Brian L. Swick [Citation ends].

Metabolic causes
Blistering diseases may be caused by errors in metabolism (e.g., nutritional and enzyme deficiencies, impaired metabolism, and endocrine abnormalities).
Nutritional deficiencies of zinc (acrodermatitis enteropathica), biotin, and essential fatty acids may present as vesicular-bullous dermatitis. These deficiencies may be inherited or acquired.
Niacin (vitamin B3) deficiency, or pellagra, is a chronic disease affecting the gastrointestinal (GI) tract, nervous system, and skin.[1] Pellagra can be inherited or acquired.
Porphyria cutanea tarda is a rare metabolic blistering disease in which the activity of the heme synthetic enzyme uroporphyrinogen decarboxylase is deficient.
Pseudo-porphyria is a condition seen in patients who are either on dialysis for chronic renal failure or are treated with drugs such as non-steroidal anti-inflammatory drugs, furosemide, nalidixic acid, and tetracycline.[21]
Diabetic bullae (bullosis diabeticorum) are non-inflammatory, spontaneous, painless blisters that typically occur in acral locations in patients with a long-standing history of diabetes.
Bullous lesions occur in approximately 40% of patients with amyloidosis.[1] Primary systemic amyloidosis is a plasma cell dyscrasia that results in over-production and deposition of insoluble monoclonal immunoglobulin light chains in mesenchymal tissue, the heart, tongue, GI tract, and skin, and can cause cutaneous amyloidosis and blisters.
Genetic causes
Incontinentia pigmenti is an X-linked dominant condition caused by mutations in the kappa kinase nuclear essential modifier (NEMO) gene, and is characterised by patterned pigmentation on the trunk followed by vesicular and verrucous forms.[1]
Bullous ichthyosiform erythroderma, also referred to as epidermolytic hyper-keratosis, is a rare autosomal-dominant congenital ichthyosis that presents with generalised erythema and superficial blisters due to a defect in keratins 1 and 10.[22]
Inflammatory causes
Eczematous dermatitis (e.g., allergic contact, nummular, and dyshidrotic dermatitis) corresponds to application of an offending agent or contact with an allergen such as poison ivy, and presents with vesicles and bullae in the acute stages of disease.
Mastocytosis is a heterogeneous group of disorders, characterised by clonal mast cell proliferation in which oedema, urtication, vesicles, and bullae may be seen.[23][Figure caption and citation for the preceding image starts]: Mastocytosis (urticaria pigmentosa)Courtesy of Fu-Tong Liu, MD [Citation ends].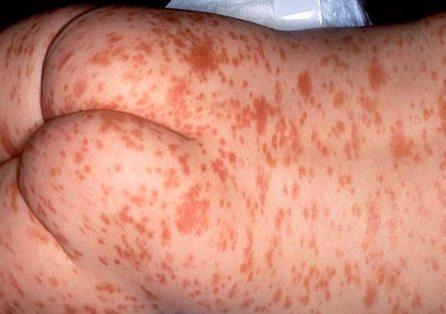
Extrinsic causes
Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis are inflammatory skin conditions. Most cases are due to exposure to medications, but bacterial and viral triggers are also common.[24] All are considered variants of a common systemic process of cutaneous epidermal necrosis with and without mucosal involvement. Designation depends on the extent of involvement of body surface area.[21]
Thermal burns may result in vesicles and bullae depending on the severity of the burn.
Frostbite refers to soft tissue that is frozen and locally deprived of its blood supply. It presents with various degrees of vesicles and bullae accompanying tissue injury.
Bullous arthropod bite reactions occur in sensitised people as a delayed hypersensitivity immune response.[25][Figure caption and citation for the preceding image starts]: Palmar target lesionsFrom the collection of Nanette Silverberg, MD [Citation ends]. [Figure caption and citation for the preceding image starts]: Target and targetoid lesionsFrom the personal collection of Nanette Silverberg, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Target and targetoid lesionsFrom the personal collection of Nanette Silverberg, MD [Citation ends].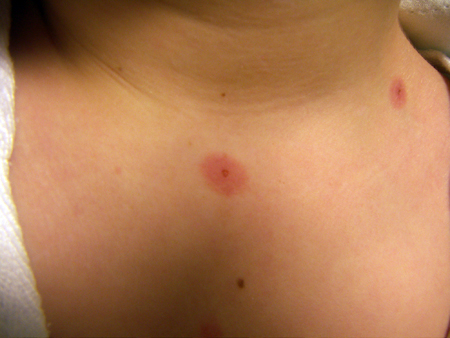 [Figure caption and citation for the preceding image starts]: Bullous erythema multiforme (EM)Courtesy of Daniel Eisen, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Bullous erythema multiforme (EM)Courtesy of Daniel Eisen, MD [Citation ends]. [Figure caption and citation for the preceding image starts]: Bullous erythema multiforme (EM)Courtesy of Daniel Eisen, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Bullous erythema multiforme (EM)Courtesy of Daniel Eisen, MD [Citation ends].
Autoimmune causes
Autoimmune bullous diseases (acquired chronic disorders associated with auto-antibodies against the structural components that maintain cell-cell and cell-matrix adhesion in the skin and mucous membrane) are divided into 2 major categories, depending on whether the blistering is intraepidermal (e.g., pemphigus) or subepidermal (e.g., pemphigoid).[26]
Pemphigus (intraepidermal blistering) is a rare group of autoimmune diseases with 3 major subsets:
Pemphigus vulgaris (PV): associated antigen is a 130-kD protein, desmoglein 3 transmembrane adhesion molecule.[26][27] This is the most common form, typically occurring in the fifth and sixth decades of life, with a predilection for those of Jewish or Mediterranean descent.[28]
Pemphigus foliaceus (PF): associated antigen is a 165-kD protein, desmoglein 1. This is the same antigen that is degraded in staphylococcal scalded skin syndrome.[7][27] PF has 2 other variants: fogo selvagem (endemic in Brazil) and pemphigus erythematosus (an overlap with lupus erythematosus).
Paraneoplastic pemphigus (PNP): develops in the context of a known or occult neoplasm (e.g., non-Hodgkin's lymphoma, chronic lymphocytic leukaemia, Castleman's disease, thymoma, Waldenstrom's macroglobulinaemia, and spindle cell sarcoma).[26][Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends].
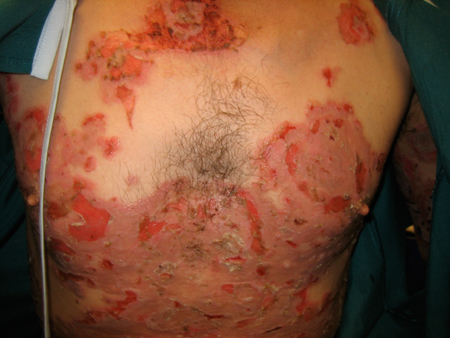 [Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends]. [Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends].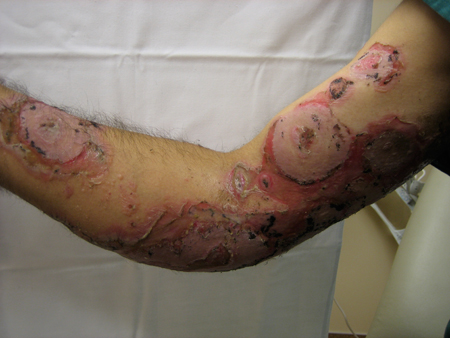 [Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Pemphigus vulgaris (PV)Courtesy of April Armstrong, MD [Citation ends].
Drug-induced pemphigus often does not resolve when the drug is withdrawn. Once induced, it behaves like acquired idiopathic pemphigus (PV, PF, PNP).[26] Typical drugs implicated in drug-induced pemphigus include penicillamine, captopril, penicillin, ceftazidime, beta-blockers, pyrazole compounds, progesterone, rifampicin, and heroin.[21][27]
The pemphigoid group of diseases are characterised by separation at the dermal-epidermal junction (subepidermal blistering), and are associated with auto-antibodies to structural components of the hemidesmosome-anchoring filaments-anchoring fibril complex.[29] Bullous pemphigoid (BP) is the most common. BP is associated with certain medications, particularly immune checkpoint inhibitors used for various cancers.[30][31] Pemphigoid gestationis occurs during the late second or third trimester of pregnancy and typically resolves on delivery. Immunologically, this form is indistinguishable from BP.[26] Mucous membrane pemphigoid (formerly cicatricial pemphigoid) may result in blindness and stenosis of laryngeal and pharyngeal mucosa.
Epidermolysis bullosa acquisita (EBA) is characterised by cell-poor or cell-rich subepidermal blisters and tissue-bound and circulating auto-antibodies to the dermal-epidermal junction.[29] Type VII collagen, the main constituent of anchoring fibrils located beneath the lamina densa, is the target auto-antigen.
Bullous lupus erythematosus (BLE) and EBA share clinical and histopathological features, as well as anti-basement membrane zone antibodies to type VII collagen. Key features that distinguish EBA from BLE include: BLE occurs in patients with a diagnosis of systemic lupus established by American College of Rheumatology criteria; sun-exposed skin is preferentially involved in BLE.[1]
Dermatitis herpetiformis is an intensely pruritic, chronic autoimmune blistering skin disease characterised by subepidermal bullae with neutrophilic micro-abscesses in the papillary dermis, granular IgA deposits in the papillary dermis on direct immunofluorescence, and an association with coeliac disease.[1][27][29]
Linear IgA bullous dermatosis (LAD) is a subepidermal blistering disorder characterised by subepidermal bullae with collections of neutrophils in the dermal papillae, as well as a circulating IgA anti-basement membrane zone antibody with broad, linear basement membrane zone deposits of IgA on direct immunofluorescence.[1] It can be induced by vancomycin, but is generally idiopathic.
Childhood linear IgA disease (chronic bullous disease of childhood) is an acquired self-limited disease with immunopathological findings similar to LAD.
Use of this content is subject to our disclaimer