Approach
Patients may present with focal neurological deficits according to the tumour location, or with signs of elevated intracranial pressure (e.g., headache, diminished consciousness, or nausea and vomiting). However, it can be difficult to distinguish clinically between the different gliomas, between these and other types of brain tumour, or between tumours and more common benign neurological conditions that present with similar signs and symptoms.[20] Rapidly progressive neurological symptoms may, however, reflect a more aggressive course.
When a brain tumour is suspected, confirmatory brain imaging with contrast-enhanced magnetic resonance imaging should be done. Ultimately, tissue obtained from a biopsy or surgical resection is required for diagnosis, based on both histopathological and molecular information.[20][21][22][23][24]
Neurological deficits according to location
Frontal:
Personality change
Cognitive decline
Emotional lability
Non-fluent aphasia (if dominant inferior frontal gyrus involved)
Motor weakness (precentral gyrus)
Temporal:
Visual field deficits
Fluent aphasia (if dominant lobe involved)
Parietal:
Sensory deficits
Dominant side inferior parietal lobule: expressive aphasia
Superior: Gerstmann's syndrome (acalculia, finger agnosia, left-right confusion, alexia without agraphia)
Non-dominant side: neglect, anosognosia
Visual field deficits
Occipital:
Contralateral homonymous hemianopsia
Cerebellum:
Vermis: truncal ataxia
Hemisphere: ipsilateral dysmetria, nystagmus
Brainstem:
Cranial nerve (CN) palsies
CN III, IV, VI: diplopia
CN V: facial sensory disturbances
CN VII: facial palsy
CN VIII: hearing or balance problems
CN IX: sensory problems in throat, swallowing problems
CN X: voice change (i.e., hoarseness), swallowing problems
CN XI: weakness of sternocleidomastoid or trapezium muscles
CN XII: deviation of the tongue
Nystagmus
Cerebellar signs
Long tract signs
Optico-hypothalamic lesions:
Visual field deficits
Excess or deficiency in pituitary hormones
Hypothalamic syndrome
Spinal cord (findings below the level of the lesion)
Motor (ipsilateral weakness, hyper-reflexia, clonus, spasticity)
Sensory (ipsilateral light touch, proprioceptive, vibration deficits/contralateral pain and temperature deficits)
Autonomic changes
Cord syndromes (central, Brown-Sequard's, posterior, anterior)
Dorsal pain
Radicular pain
Sphincter disturbances
Seizure
One review reported that 75% to 80% of patients with low-grade gliomas have seizures, compared with 29% to 60% of patients with glioblastoma.[25]
Intracranial hypertension and vasogenic oedema
Patients may present with headache, nausea, vomiting, diplopia, and/or decreased level of arousal. They may also have signs of intracranial hypertension (sixth [VI] nerve palsy and papilloedema).
Laboratory evaluation
Hypothalamic-pituitary axis hormones should be ordered if there is a known hypothalamic or pituitary region lesion, or the patient has symptoms or signs suggestive of hypopituitarism.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) with gadolinium is the imaging modality of choice for the evaluation of gliomas, before and after biopsy or surgical resection, and as a surveillance method to detect the progression of previously treated tumours.[21][22][24][26][27][28][29]
With high-grade gliomas, areas of gadolinium enhancement correspond with regions of increased vascularity or endothelial proliferation, and increased blood-brain barrier permeability. Areas of increased T2-fluid-attenuated inversion recovery (FLAIR) signal often correspond to non-necrotic, infiltrating areas of the tumour, although they can also be associated with peritumoural oedema or radiation-induced white matter changes. Low-grade (grade 2) isocitrate dehydrogenase (IDH)-mutant gliomas are most commonly non-enhancing and are detected by areas of increased T2-FLAIR signal.[21][22]
Gliomas are intra-axial lesions frequently centred in, or extending along, white matter tracts.[Figure caption and citation for the preceding image starts]: T2-weighted MRI without (A) and with (B) contrast demonstrating a pontine gliomaFrom the collection of Karine Michaud, University of California, San Francisco [Citation ends].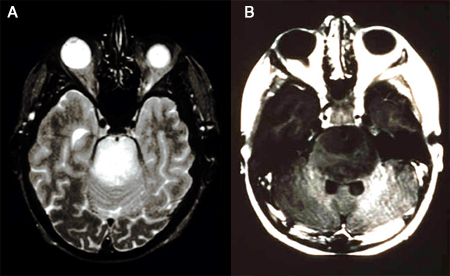 [Figure caption and citation for the preceding image starts]: MRI: T2 and T1 post-contrast, demonstrating a tectal glioma (grade 2)From the collection of Karine Michaud, University of California, San Francisco [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI: T2 and T1 post-contrast, demonstrating a tectal glioma (grade 2)From the collection of Karine Michaud, University of California, San Francisco [Citation ends].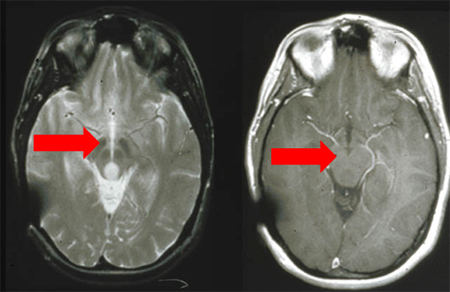 [Figure caption and citation for the preceding image starts]: MRI demonstrating a right temporal glioblastoma (grade 4)From the collection of Karine Michaud, University of California, San Francisco [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI demonstrating a right temporal glioblastoma (grade 4)From the collection of Karine Michaud, University of California, San Francisco [Citation ends].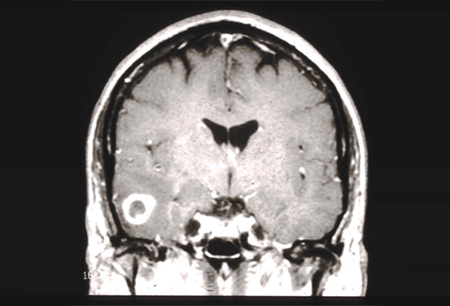 [Figure caption and citation for the preceding image starts]: MRI demonstrating a right frontal grade 2 diffuse astrocytomaFrom the collection of Karine Michaud, University of California, San Francisco [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI demonstrating a right frontal grade 2 diffuse astrocytomaFrom the collection of Karine Michaud, University of California, San Francisco [Citation ends].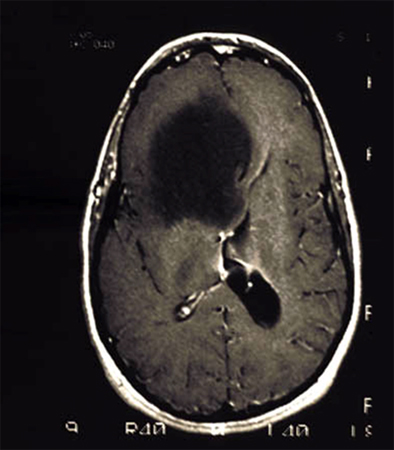 [Figure caption and citation for the preceding image starts]: MRI demonstrating a cerebellar pilocytic astrocytoma (grade 1)From the collection of Karine Michaud, University of California, San Francisco [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI demonstrating a cerebellar pilocytic astrocytoma (grade 1)From the collection of Karine Michaud, University of California, San Francisco [Citation ends].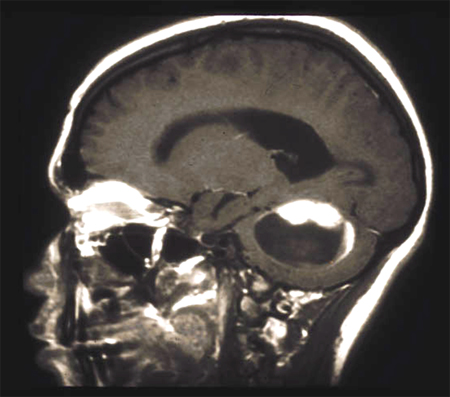
MR spectroscopy (MRS) is frequently employed to interrogate selected areas of increased T2-FLAIR signal for specific metabolites.[21][22][27] It is used to differentiate between normal and neoplastic tissue, and to assist with diagnosis, such as when the peak corresponding to 2-hydroxyglutarate, the metabolite resulting from the activity of mutant IDH, is detected. MRS can also be used to differentiate between true tumour progression and treatment-related imaging changes or pseudo-progression in patients with suspected progressive glioblastoma.[26][27]
N-acetylaspartate: highest peak in the normal brain, neuronal marker.
Choline: marker of cell membrane turnover; increases in this peak are associated with malignancy.
Lactate: marker of hypoxia; increases in this peak are associated with malignancy.
Diffusion tensor MRI produces directionally encoded maps of the cerebral white matter tracts.[21] Tractography is commonly used in preoperative planning to identify important subcortical structures such as the corticospinal tract (motor function) and arcuate fasciculus (language function).
Perfusion MRI provides quantitative assessment of regional cerebral blood flow and cerebral blood volume, reflecting the magnitude of angiogenesis in a tumour, thus helping to differentiate tumour progression from radiation-induced changes in enhancing areas of previously treated tumours.[21]
Functional MRI is the study of regional changes in blood flow related to function and is used in preoperative planning for tumours in eloquent locations (areas of the brain that control speech, motor function, and senses).[21]
Computed tomography
Computed tomography (CT) can be a useful imaging modality for evaluating gross changes in tumour size, or the presence of intratumoural haemorrhage.
CT should not be used routinely in the diagnosis and surveillance of brain tumours because of the superior resolution and imaging quality of MRI.[21] It may be used if MRI is unavailable or contraindicated. CT may be ordered if the presentation is acute and there is a need to rule out other processes such as herniation, stroke, and haemorrhage.
Ophthalmological evaluation
Ophthalmological evaluation, including visual field testing, may be ordered if there are symptoms or signs of visual loss or evidence of compression of the optic chiasm on MRI.
Histopathology investigations
Resection or biopsy to obtain tissue for histopathological analyses should be carried out for all patients.
If the tumour is deemed accessible, maximal safe resection is attempted unless frozen pathology results reveal primary central nervous system lymphoma or lesions with non-neoplastic aetiologies. For tumours that are inaccessible to resection, or if the patient is not deemed medically fit for a lengthy operation, a stereotactic or open biopsy should be performed.[21][22] Tissue samples are sent for histological and molecular analyses prior to initiating further management.
Molecular analyses
Molecular characterisation of tissue obtained from resection or biopsy is essential for accurate classification and to guide subsequent treatment decisions. Molecular markers that should be investigated (depending on clinical context, frozen histology results, and imaging findings) include IDH1 and IDH2 mutation, 1p/19q-codeletion, CDKN2A/B deletion, ATRX mutation, TP53 mutation, MGMT promoter methylation status, EGFR amplification, chromosome 7 gain, chromosome 10 loss, TERT promoter mutation, and histone H3 mutation.[1][21][22][23][24][27][30]
[Figure caption and citation for the preceding image starts]: Important molecular tests to establish the diagnosis and prognosis of glioblastomaGritsch et al. Cancer. 2022 Jan 1;128(1):47-58; used with permission [Citation ends].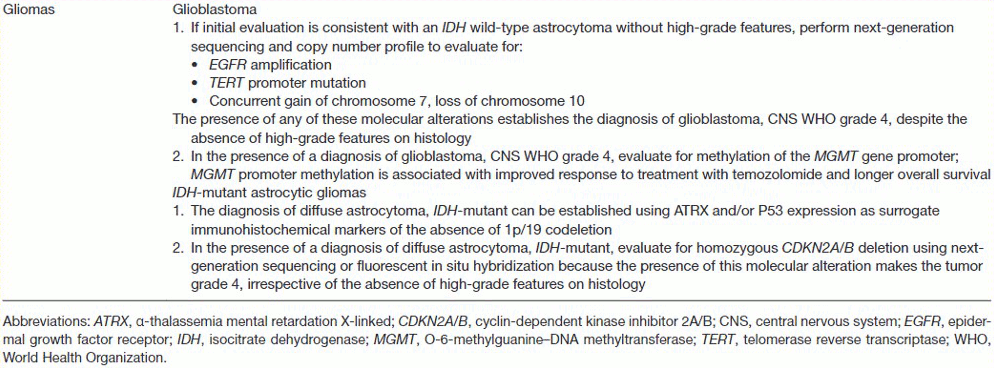
Use of this content is subject to our disclaimer