History and exam
Key diagnostic factors
common
presence of risk factors
Key risk factors include increasing age, white or black ethnicity (compared with Latin Americans and Native Americans), high socioeconomic class, positive family history, prolonged oestrogen/progestin exposure, high levels of alcohol consumption, history of radiation exposure, existing benign breast disease and increased breast density.
uncommon
breast mass
The clinician should elicit whether the breast mass is tender, if there are changes in the size or character of the mass, and whether the characteristics of the mass have been affected by the menstrual cycle.
It should be remembered that breast cancers do not always present with a new breast mass. Many breast cancers are diagnosed on the basis of mammographic abnormalities (such as linear or pleomorphic microcalcifications), in the absence of a palpable mass.[102]
Occult breast cancer is found in approximately 0.3% of women diagnosed with axillary lymphadenopathy.[128]
nipple discharge
May be watery, serous, milky, or bloody. While bloody discharge is more classically associated with a neoplasm, it may also be related to an intraductal papilloma. The relation of nipple discharge to malignancy appears to be affected by age. Among women with nipple discharge, cancer was present in 3% who were under 40 years, 10% who were aged 40-60 years, and 32% who were over 60 years.[159]
axillary lymphadenopathy
Axillary nodal involvement is the most reproducible prognostic factor for primary invasive breast cancer. The probability of axillary nodal involvement increases in proportion to the size of the tumour.[160] However, this positive linear relationship may not apply to tumours <1 cm and those >5 cm.[161] Clinical assessment of nodal status can often be inaccurate; therefore, imaging (e.g., CT scan) is required.[129]
Other diagnostic factors
uncommon
skin thickening or discoloration
Skin is involved in 20% of all cases of breast cancer.[127] However, skin changes, such as peau d'orange (dimpling of the skin), erythema, and ulceration, are always associated with locally advanced or inflammatory breast cancer.[Figure caption and citation for the preceding image starts]: Peau d'orangeFrom the collection of Dr Amal Melhem-Bertrandt; used with permission [Citation ends].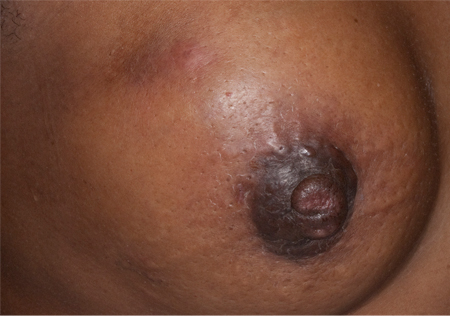
retraction, inversion, or scaling of the nipple
May be related to Paget's disease of the breast or locally advanced breast cancer in association with a large breast mass with/without nipple or skin involvement.[Figure caption and citation for the preceding image starts]: Nipple retraction and asymmetryFrom the collection of Dr Amal Melhem-Bertrandt; used with permission [Citation ends].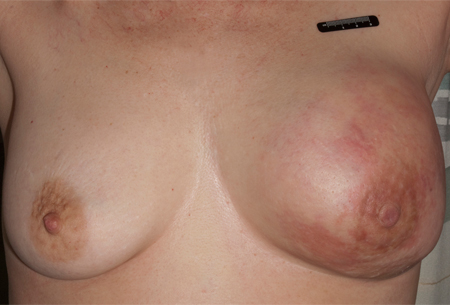
Risk factors
strong
increasing age
The likelihood of breast cancer rises markedly with increasing age during the first 6 decades of life.[8]
Of women diagnosed with breast cancer between 2017 and 2021 in the US, approximately 2.0% were diagnosed between 20 and 34 years; 8.4% between 35 and 44 years; 18.2% between 45 and 54 years; 25.1% between 55 and 64 years; 27.1% between 65 and 74 years; 14.2% between 75 and 84 years; and 5.0% >84 years of age.[8]
The median age at diagnosis for breast cancer in women in the US is 63 years.[8]
female sex
In the US, the incidence of breast cancer is approximately 100 times greater in women than in men.[7]
Insufficient evidence exists to determine whether transgender people undergoing hormone therapy have an overall lower, average, or higher risk of developing breast cancer compared with birth-sex controls.[29]
ethnic origin
In the US, the age-adjusted incidence of female breast cancer (based on data from 2017 to 2021) is highest in non-Hispanic white women (139.0 per 100,000), followed by non-Hispanic black women (129.3 per 100,000), non-Hispanic American Indian/Alaska Native women (113.0 per 100,000), non-Hispanic Asian/Pacific Islander women (110.3 per 100,000), and Hispanic women (101.2 per 100,000).[8]
Age-adjusted mortality rates in non-Hispanic black women remain higher than in white women (26.8 per 100,000 vs. 19.4 per 100,00 [based on data from 2018 to 2022]).[8] This may be related to a more advanced stage at initial diagnosis, disparities in health care access and treatment, higher tumour grade, and greater incidence of oestrogen receptor-negative tumours in black patients.[30] Immigrant and minority ethnic women are less likely to attend mammographic screening compared with other women, potentially leading to a delay in breast cancer diagnosis.[31]
A study of the National Program of Cancer Registries and US Cancer Statistics noted that the prevalence of triple-negative breast cancer among black women in the US varies by birthplace (e.g., Western-African-born, Caribbean-born, or Eastern-African-born).[32]
BRCA1 and BRCA2 germline mutations are the most common inherited genetic mutation found in breast cancer.[14][15][16] However, in Ashkenazi Jews, there is a higher population frequency (approximately 2%) of three founder mutations: BRCA1 185delAG, BRCA1 5382insC, and BRCA2 6174delT.[33]
positive family history
genetic mutations
It is estimated that 5% to 10% of breast cancers are linked to inherited genetic mutations.[12][13][14] BRCA1 and BRCA2 mutations are the most common inherited genetic mutation found in breast cancer.[14][15][16]
BRCA1-related breast tumours have less tubule formation, more pleomorphism, and a higher mitotic count compared with sporadic breast cancer tumours.[36] BRCA1-associated breast cancers occur at an early age, and are more likely to be oestrogen receptor-negative and progesterone receptor-negative.[25][37][38]
BRCA2-related breast tumours have less tubule formation compared with sporadic breast cancer tumours, but pleomorphism and mitotic count are similar.[36] BRCA2 mutations are associated with early onset breast and ovarian cancer, male breast cancer, prostate cancer, and pancreatic cancer.[26][39]
In a large international prospective cohort study, the cumulative risk of breast cancer was reported to be 72% and 69% for BRCA1 and BRCA2 germline mutation carriers, respectively.[16] The cumulative risk for contralateral breast cancer 20 years after initial breast cancer diagnosis was reported to be 40% and 26% for BRCA1 and BRCA2 germline mutation carriers, respectively.[16]
BRCA-related breast cancers are more frequent in the Ashkenazi Jewish population due to the higher frequency of BRCA mutations in this population (exceeding 2%) compared with the general population.[33]
Other genetic mutations associated with breast cancer include: CHEK2, PALB2, ATM, NBN, BARD1, RAD51C, RAD51D, CDH1 (hereditary diffuse gastric cancer), NF1 (neurofibromatosis type 1), PTEN (Cowden syndrome), STK11 (Peutz-Jeghers syndrome), and TP53 (Li-Fraumeni syndrome).[18]
A deletion mutation in the checkpoint kinase 2 gene (CHEK2*1100delC), which introduces a premature stop codon, is associated with increased breast cancer risk in female and male patients.[40] The CHEK2*1100delC allele is more prevalent in people of Northern European descent and is present in 5% of non-BRCA1/BRCA2 breast cancer families, compared with 1% of the general population.[40]
In a study of women who had genetic testing in two US states, prevalent pathogenic variants in patients with breast cancer were BRCA1 (3.2%), BRCA2 (3.1%), CHEK2 (1.6%), PALB2 (1.0%), ATM (0.7%), and NBN (0.4%).[41]
endogenous oestrogen exposure
Prospective trials have demonstrated the correlation between increased levels of endogenous sex hormones and a significant elevation in the risk of breast cancer.[19][20]
The risk of breast cancer decreases with an older age of menarche and younger age of menopause. Average breast cancer risk increases by 4.0% with each year of earlier-onset menarche, and by 3.6% for each year of later-onset menopause.[42] These risk factors have a stronger effect on the incidence of oestrogen receptor-positive breast cancers than on oestrogen receptor-negative breast cancers.[43]
Pregnancy and breastfeeding reduce cumulative exposure to endogenous hormones, and are associated with decreased breast cancer risk.[44][45] However, pooled analysis of data from prospective studies indicated an increased risk of breast cancer (mainly oestrogen receptor-positive) in parous women for up to 20 years following childbirth compared with nulliparous women.[46] Risk was more pronounced among women with a family history of breast cancer, those older at first birth, and those who had more births. With longer follow-up, the anticipated inverse association between childbirth and oestrogen receptor-positive breast cancer was evident.[46]
exogenous oestrogen/progestin exposure
Women exposed to exogenous oestrogen and progestin (e.g., via hormone replacement therapy [HRT] or hormonal contraception) have an increased risk of breast cancer.[21][22][23]
The US-based Women's Health Initiative (WHI) comprised two placebo-controlled randomised clinical trials involving 27,347 post-menopausal women. Interventions were conjugated equine oestrogens with medroxyprogesterone acetate for women with an intact uterus (n = 16,608) and conjugated equine oestrogens alone for women with prior hysterectomy (n = 10,739). The trial that randomised post-menopausal women to combined oestrogen plus progestin HRT or placebo was stopped early when it reported that post-menopausal women in the oestrogen plus progestin-containing HRT arm of the trial had a 26% increased risk of breast cancer, a 29% increased risk of coronary heart disease, a 41% increased risk of stroke, and a 213% increased risk of pulmonary embolism, after a mean of 5.2 years of follow-up.[21] In the parallel WHI trial, use of oestrogen alone significantly reduced breast cancer incidence in women with prior hysterectomy.[47] The hazard ratio (HR) for invasive breast cancer risk was lower than 1 throughout the intervention phase (HR 0.79, 95% CI 0.61 to 1.02) and remained lower than 1 in the early post-intervention phase (HR 0.55, 95% CI 0.34 to 0.89), but risk reduction was not observed during the late post-intervention follow-up (HR 1.17, 95% CI 0.73 to 1.87).[47]
After more than 20 years of median cumulative follow-up, the WHI concluded that the use of oestrogen and progestin by women with a uterus was associated with a significant increase in breast cancer incidence (annualised rate 0.45%), compared with women taking placebo (annualised rate 0.36%). However, use of oestrogen and progestin was not associated with increased breast cancer mortality.[48]
One prospective cohort study carried out in Denmark reported a 20% increased risk of breast cancer in women who were current users or who had recently used hormonal contraception (combined oestrogen and progestin, and progestin-only), compared with never-users.[22] Risk increased with longer use of hormonal contraception.[22]
Based on the available literature on the use of exogenous oestrogen and progestin, the US Preventive Services Task Force has concluded that the harmful effects of combined oestrogen and progestin are likely to exceed the chronic disease prevention benefits in most women; therefore, its use is not recommended for the primary prevention of chronic conditions in post-menopausal women.[49][50]
alcohol consumption
Moderate levels of alcohol intake are associated with an increased risk of breast cancer, and this risk increases with higher levels of alcohol consumption.[51][52][53] Alcohol consumption should ideally be avoided. People who choose to drink alcohol should limit their intake to no more than one drink equivalent in one day and no more than three per week.[54]
The effect of alcohol consumption may be mediated by increasing oestrogen levels. Post-menopausal women who consume high levels of alcohol demonstrate a higher incidence of oestrogen receptor-positive breast cancers.[55] Other studies have indicated that folic acid may reduce the effect of alcohol on breast cancer risk.[56]
radiation exposure
Radiotherapy for primary invasive breast cancer may increase the risk of contralateral breast cancer >5 years after treatment.[57] Repeated fluoroscopic examinations, such as those previously used to monitor for tuberculosis, also increase the risk of breast cancer.
One modelling study found that screening female childhood cancer survivors with annual MRI, with or without mammography, from age 25 years could prevent up to 71% of deaths from breast cancer in this group. Without screening, the lifetime breast cancer mortality risk was 11%.[58]
Mammography is a low-dose, low-energy radiation exposure with a minimal effect on breast cancer risk.[59]
Atomic bomb survivors and patients who received radiotherapy with the mantle technique have an increased risk of breast cancer.[60][61] The adolescent and young adult breast appears to be most susceptible, but the risk of breast cancer may still be elevated in women who are exposed to radiation at ages up to 45 years.[62]
atypical breast disease
Studies have reported that women with benign breast disease have a relative risk of 1.5 to 1.6 for developing breast cancer compared with women in the general population.[63][64][65] The increased risk is clinically significant in those with atypical breast disease; relative risk increases to 1.88 or 4.24 if histology shows hyperplasia without atypia or hyperplasia with atypia, respectively.[65]
increased breast density
A case-control study found that women with a mammographic density of ≥75% had an odds ratio of 4.7 for developing breast cancer compared with women with mammographic density <10%.[66] This elevated risk remained for at least 8 years after study entry, and the difference was more prominent in younger women.
In a longitudinal study of mammographic density, an increase in density within 3 years was associated with an increase in breast cancer risk.[67]
A large cohort study of women screened by the US Breast Cancer Surveillance Consortium found high advanced breast cancer diagnosis rates (≥0.61 cases per 1000 mammograms) within 12 months of screening mammography in: women with heterogeneously dense breasts, and a 5-year breast cancer risk of 2.5% or higher; and women with extremely dense breasts and a 5-year breast cancer risk of 1% or higher.[68][69] Women in these groups may derive most benefit from discussion about breast density and supplemental imaging.
mild, moderate, or marked background parenchymal enhancement (BPE) on breast MRI
BPE refers to the uptake of gadolinium-based contrast by normal breast tissue on breast MRI. It may represent increased tissue micropermeability and/or vascularity.
One observational study of 4247 women undergoing screening and diagnostic breast MRI found that women with mild, moderate, or marked BPE had an increased risk of invasive breast cancer, compared with women with minimal BPE (hazard ratio [HR] 2.73, 95% CI 1.66 to 4.49). Increasing levels of BPE were associated with increased risk.[70]
In one retrospective meta-analysis of observational studies (including 1910 women with breast cancer and 2541 control participants), a higher level of BPE was associated with the presence of breast cancer among women with high risk for breast cancer, but not in women with average risk.[71]
weak
reduced physical activity
Sedentary behaviour is associated with increased breast cancer risk.[72][73][74][75]
In post-menopausal women, physical activity and sustained weight loss have been associated with reduced risk of breast cancer.[76][77] The ability of physical exercise to reduce endogenous oestrogen levels has been postulated as the mechanism underlying this effect.
In pre-menopausal women, some studies have demonstrated that regular physical activity reduces the risk of early-onset breast cancer, while others have shown that exercise has no effect on the incidence of breast cancer.[78][79][80] One meta-analysis of observational cohort studies found an inverse association between greater physical activity and pre-menopausal and post-menopausal breast cancer incidence.[73]
Using Mendelian randomisation to simulate a randomised controlled trial, vigorous physical activity ≥3 days per week was associated with a 38% reduced risk of breast cancer in pre- and peri-menopausal women compared with no vigorous activity.[81]
poor diet
Studies evaluating the effect of diet on breast cancer risk have yielded inconclusive or inconsistent results.[82] In the NIH-AARP Diet and Health Study, patients in the highest quintile of animal fat intake were found to have an increased risk of developing breast cancer.[83][84]
Systematic reviews and meta-analyses have found that fruit, vegetable, and fatty fish consumption lower the risk of breast cancer.[85][86] Randomised controlled trials of a reduced-fat diet did not result in reduced breast cancer risk.[87][88]
high socioeconomic status
Breast cancer is diagnosed more often in patients from a higher socioeconomic class and those who have a higher level of education.[89] This relationship may stem from differences in lifestyle, such as age at birth of first child and exogenous hormone use, that may increase the risk of breast cancer in this population.
Low socioeconomic status is associated with increased risk of aggressive pre-menopausal breast cancer, later stage at diagnosis, and worse survival.[90]
smoking
obesity
Obesity is associated with an increased risk of breast cancer in post-menopausal women and a decreased risk of breast cancer in pre-menopausal women.[95][96]
The association between BMI and breast cancer incidence is stronger in Asia-Pacific populations (relative risk [RR] 1.18) than in European-Australian or North American populations.[97] Each 5 kg weight gain in adulthood increases the risk of post-menopausal breast cancer in women who have never used hormone replacement therapy by 11%.[98]
One retrospective cohort study reported a reduced risk of breast cancer amongst pre-menopausal and post-menopausal women who had bariatric surgery (for BMI ≥35 kg/m²) compared with women of the same BMI who did not have bariatric surgery.[99]
high dibutyl-phthalate exposure
One small case-control study reported a significant association between urinary dibutyl-phthalate concentration and increased risk for breast cancer.[100]
One Danish cohort study identified a two-fold increased risk in the rate of oestrogen receptor-positive breast cancer in women exposed to high (≥10,000 mg) cumulative doses of dibutyl-phthalate in medication. Lower levels of exposure were not associated with an increased incidence of breast cancer.[101]
Phthalates may promote breast tumour growth through oestrogen receptor signalling. They are constituents in packaging, cosmetics, personal care, and household items. Dibutyl-phthalates are used in pharmaceutical capsules to impart delayed or extended-release properties.
Use of this content is subject to our disclaimer