Breast cancer may present as a painless firm breast mass, especially when discovered in the early stages. Factors that support the diagnosis include:
Increasing size of the mass (fibrocystic disease may fluctuate in size with the menstrual cycle, but breast cancer progressively increases in size regardless of the menstrual cycle)
Nipple discharge
Axillary lymphadenopathy
Skin thickening or discoloration (more likely to be associated with locally advanced or inflammatory breast cancer)[127]Ho CM, Mak CK, Lau Y, et al. Skin involvement in invasive breast carcinoma: safety of skin-sparing mastectomy. Ann Surg Oncol. 2003 Mar;10(2):102-7.
http://www.ncbi.nlm.nih.gov/pubmed/12620902?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Peau d'orangeFrom the collection of Dr Amal Melhem-Bertrandt; used with permission [Citation ends].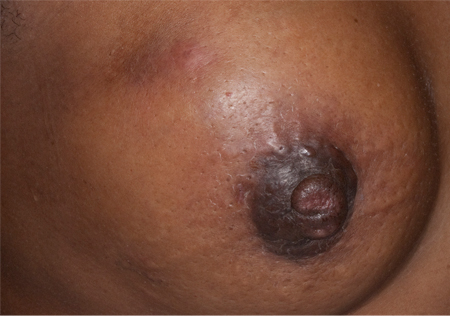
Retraction, inversion, or scaling of the nipple (may be related to Paget's disease of the breast, or locally advanced breast cancer in association with a large breast mass with/without nipple or skin involvement).[Figure caption and citation for the preceding image starts]: Nipple retraction and asymmetryFrom the collection of Dr Amal Melhem-Bertrandt; used with permission [Citation ends].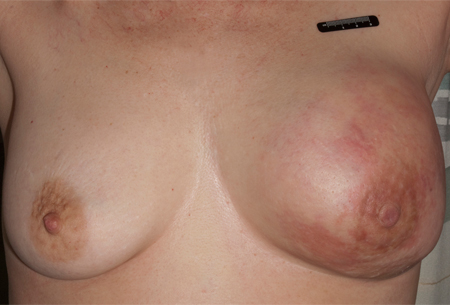
It should be remembered that breast cancers do not always present with a new breast mass. Many breast cancers are diagnosed on the basis of mammographic abnormalities (such as linear or pleomorphic microcalcifications), in the absence of a palpable mass.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
Occult breast cancer is found in approximately 0.3% of women diagnosed with axillary lymphadenopathy.[128]Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys. 2000 Apr 1;47(1):143-7.
http://www.ncbi.nlm.nih.gov/pubmed/10758316?tool=bestpractice.com
A family history should be taken, paying particular attention to close blood relatives with diagnoses of breast, ovarian, or pancreatic cancer, or metastatic, high-risk, or very-high-risk prostate cancer, to identify patients at increased genetic/familial risk of breast cancer.[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
Genetic risk evaluation, including counselling and genetic testing, is recommended for patients with a strong family history or a known or likely pathogenic variant in a cancer susceptibility gene.[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
Physical examination
The patient should be first examined in the sitting position to allow inspection for changes in skin colour, contour, dimpling, and asymmetry. The axilla is also evaluated by relaxing and adducting the arm. The supraclavicular and infraclavicular nodal basins should be evaluated to determine the extent of nodal disease.
Clinical assessment of nodal status is often inaccurate; therefore, imaging (e.g., ultrasonography, contrast-enhanced breast magnetic resonance imaging [MRI], or computed tomography [CT]) is required. A study by the National Surgical Adjuvant Breast Project (NSABP) found histological evidence of nodal metastases on pathological examination in 38% of a group of breast cancer patients who had been determined to be node-negative by clinical examination.[129]Fisher ER, Gregorio RM, Fisher B, et al. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1-85.
https://acsjournals.onlinelibrary.wiley.com/doi/pdf/10.1002/1097-0142%28197507%2936%3A1%3C1%3A%3AAID-CNCR2820360102%3E3.0.CO%3B2-4
http://www.ncbi.nlm.nih.gov/pubmed/173455?tool=bestpractice.com
Conversely, no evidence of nodal disease was later found on pathological examination in 25% of the patients who had been clinically determined to have nodal metastases.[129]Fisher ER, Gregorio RM, Fisher B, et al. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1-85.
https://acsjournals.onlinelibrary.wiley.com/doi/pdf/10.1002/1097-0142%28197507%2936%3A1%3C1%3A%3AAID-CNCR2820360102%3E3.0.CO%3B2-4
http://www.ncbi.nlm.nih.gov/pubmed/173455?tool=bestpractice.com
The patient is then examined supine, with their arm raised so that the hand is behind the head and the breast tissue is distributed over the chest wall, facilitating thorough palpation of the entirety of the breast and nodal regions. The tissue at and beneath the nipple should be palpated.
Physical examination of the breasts and nodal basins should follow a methodical pattern, to prevent omission of any region.
Imaging
Recommended imaging modalities include mammography, ultrasound, and MRI. Systemic staging for women with locally advanced disease or suspected metastatic disease may involve CT, bone scan, and positron emission tomography (PET)/CT.
Mammography
Bilateral diagnostic mammography should be used as the initial imaging test to evaluate symptomatic adult patients aged ≥30 years, or as follow-up to evaluate abnormal findings on screening mammography or other imaging tests.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[130]American College of Radiology. ACR practice parameter for the performance of screening and diagnostic mammography. 2023 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Screen-Diag-Mammo.pdf
[131]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
The sensitivity and specificity of conventional mammography for diagnosing breast lesions have been reported as 78.9% and 82.7%, respectively.[132]Moss HA, Britton PD, Flower CD, et al. How reliable is modern breast imaging in differentiating benign from malignant breast lesions in the symptomatic population? Clin Radiol. 1999 Oct;54(10):676-82.
http://www.ncbi.nlm.nih.gov/pubmed/10541394?tool=bestpractice.com
Digital breast tomosynthesis (DBT) is a three-dimensional mammographic technique that can be used to create thin-section reconstructed images of breast tissue. Diagnostic DBT may offer improved detection and lesion characterisation compared with conventional two-dimensional mammography, especially in patients with dense breast tissue.[130]American College of Radiology. ACR practice parameter for the performance of screening and diagnostic mammography. 2023 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Screen-Diag-Mammo.pdf
In one study, DBT showed a higher overall sensitivity, compared with conventional mammography (88.2% vs. 78.3%, respectively), with a similar specificity.[133]Kim WH, Chang JM, Moon HG, et al. Comparison of the diagnostic performance of digital breast tomosynthesis and magnetic resonance imaging added to digital mammography in women with known breast cancers. Eur Radiol. 2016 Jun;26(6):1556-64.
http://www.ncbi.nlm.nih.gov/pubmed/26376882?tool=bestpractice.com
Diagnostic conventional mammography or diagnostic DBT may be used as alternative options or in combination.[131]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
[134]Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024 Feb;35(2):159-82.
https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-breast-cancer/early-breast-cancer
http://www.ncbi.nlm.nih.gov/pubmed/38101773?tool=bestpractice.com
National Comprehensive Cancer Network (NCCN) guidelines recommend diagnostic mammography with DBT.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
Contrast-enhanced mammography with ultrasound may be a further option for initial diagnostic imaging.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[134]Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024 Feb;35(2):159-82.
https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-breast-cancer/early-breast-cancer
http://www.ncbi.nlm.nih.gov/pubmed/38101773?tool=bestpractice.com
The sensitivity of conventional mammography is lower in women with dense breasts; therefore, DBT, or supplemental ultrasound or MRI may be warranted in these women.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[135]Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and society of breast imaging. J Am Coll Radiol. 2021 Sep;18(9):1280-8.
https://www.jacr.org/article/S1546-1440(21)00383-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34154984?tool=bestpractice.com
[136]Chen HL, Zhou JQ, Chen Q, et al. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (≤2 cm) breast cancer. Medicine (Baltimore). 2021 Jul 2;100(26):e26531.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8257894
http://www.ncbi.nlm.nih.gov/pubmed/34190189?tool=bestpractice.com
Breast ultrasound
Evaluation of a new mass in a woman aged <30 years should usually begin with ultrasound (followed by mammography if results are suggestive of breast cancer).[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
However, if there is a high suspicion of malignancy (e.g., based on personal or family history, clinical breast examination, or ultrasound results), a mammogram should be performed first.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
If there is a low clinical suspicion, observation of the mass for 1-2 menstrual cycles may be considered, followed by ultrasound or mammography if symptoms persist.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
For women aged ≥30 years with palpable symptoms, and patients with other suspicious symptoms at any age, ultrasound may be performed in addition to diagnostic mammography and/or DBT.[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[131]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
[134]Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024 Feb;35(2):159-82.
https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-breast-cancer/early-breast-cancer
http://www.ncbi.nlm.nih.gov/pubmed/38101773?tool=bestpractice.com
Breast ultrasound has demonstrated utility as an adjunct to mammography through specificity (by differentiating cysts from solid masses), evaluating breast or axillary masses that are not sufficiently assessed by mammogram, evaluating axillary lymph nodal involvement, and monitoring for tumour response during neoadjuvant chemotherapy.[137]American College of Radiology. ACR appropriateness criteria: imaging of the axilla. 2021 [internet publication].
https://acsearch.acr.org/docs/3158165/Narrative
[138]American College of Radiology. ACR practice parameter for the performance of a breast ultrasound examination. 2021 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-Breast.pdf
[139]Flobbe K, Bosch AM, Kessels AG, et al. The additional diagnostic value of ultrasonography in the diagnosis of breast cancer. Arch Intern Med. 2003 May 26;163(10):1194-9.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/215601
http://www.ncbi.nlm.nih.gov/pubmed/12767956?tool=bestpractice.com
[140]Expert Panel on Breast Imaging, Hayward JH, Linden OE, et al. ACR Appropriateness Criteria® monitoring response to neoadjuvant systemic therapy for breast cancer: 2022 Update. J Am Coll Radiol. 2023 May;20(5s):S125-45.
https://acsearch.acr.org/docs/3099208/Narrative
http://www.ncbi.nlm.nih.gov/pubmed/37236739?tool=bestpractice.com
Three-dimensional breast ultrasound has a sensitivity of 92.3% and a specificity of 87.2% for diagnosing breast cancer in women with breast nodules or mass lesion.[141]Bin L, Huihui Y, Weiping Y, et al. Value of three-dimensional ultrasound in differentiating malignant from benign breast tumors: a systematic review and meta-analysis. Ultrasound Q. 2019 Mar;35(1):68-73.
https://journals.lww.com/ultrasound-quarterly/Fulltext/2019/03000/Value_of_Three_Dimensional_Ultrasound_in.11.aspx
http://www.ncbi.nlm.nih.gov/pubmed/30807546?tool=bestpractice.com
Breast MRI
Sensitivity of breast MRI is higher than for mammogram, but specificity is limited (sensitivity for breast cancer is approximately 88% to 91%; specificity approximately 68%).[142]Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004 Dec 8;292(22):2735-42.
https://jamanetwork.com/journals/jama/fullarticle/199950
http://www.ncbi.nlm.nih.gov/pubmed/15585733?tool=bestpractice.com
Despite increased sensitivity, MRI has not been shown to decrease rates of reoperation when added to the work-up of women with primary breast cancer undergoing wide local excision.[143]Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010 Feb 13;375(9714):563-71.
http://www.ncbi.nlm.nih.gov/pubmed/20159292?tool=bestpractice.com
Breast MRI is not recommended routinely for diagnostic evaluation, because of the risk of false positives and potential for overtreatment.[144]Expert Panel on Breast Imaging, McDonald ES, Scheel JR, et al. ACR appropriateness criteria: imaging of invasive breast cancer. J Am Coll Radiol. 2024 Jun;21(6s):S168-202.
https://www.jacr.org/article/S1546-1440(24)00262-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38823943?tool=bestpractice.com
Breast MRI (without and with contrast) may, however, be useful for evaluation and decision-making in certain circumstances:[102]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
[144]Expert Panel on Breast Imaging, McDonald ES, Scheel JR, et al. ACR appropriateness criteria: imaging of invasive breast cancer. J Am Coll Radiol. 2024 Jun;21(6s):S168-202.
https://www.jacr.org/article/S1546-1440(24)00262-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38823943?tool=bestpractice.com
Evaluation of suspicious nipple discharge, inversion, or retraction, or suspicious breast skin changes when ultrasound or mammography are not diagnostic. MRI may facilitate a diagnosis of inflammatory breast cancer.
Staging evaluation (to define extent of cancer or presence of multifocal or multicentric cancer), if indicated, or to screen for cancer in the contralateral breast at diagnosis.
Evaluation before and after preoperative systemic therapy (to define the extent of disease, response to treatment, and potential for breast-conserving surgery).
Identifying occult primary tumours in patients with clinically positive axillary nodes; or with Paget's disease (to define the extent of disease); or with invasive lobular carcinoma (not adequately identified on mammography, ultrasound, or physical examination).
Systemic staging
Routine systemic staging is not indicated for early-stage breast cancer in the absence of signs or symptoms of metastasis.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
In women with symptoms or signs suggestive of metastatic disease or those with locally advanced disease (T3, N1-2, M0) additional imaging should be considered.[144]Expert Panel on Breast Imaging, McDonald ES, Scheel JR, et al. ACR appropriateness criteria: imaging of invasive breast cancer. J Am Coll Radiol. 2024 Jun;21(6s):S168-202.
https://www.jacr.org/article/S1546-1440(24)00262-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38823943?tool=bestpractice.com
See Metastatic breast cancer.
Pulmonary symptoms should be investigated with chest CT (without or with contrast). Radionucleotide bone scan or sodium fluoride PET/CT scan should be performed in patients with localised bone pain or elevated alkaline phosphatase. Abdominal and pelvic imaging using CT with contrast or MRI with contrast is indicated for abdominal or pelvic symptoms or signs, elevated alkaline phosphatase, or abnormal liver function tests.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
PET/CT scan is not indicated in stage I, stage II, or operable stage III breast cancer because of its high false-negative rate for small lesions, low sensitivity for detection of axillary lymph node metastases, and high rate of false-positive scans. PET/CT is most helpful in advanced disease and invasive ductal histology when standard staging investigations are equivocal.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
[134]Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024 Feb;35(2):159-82.
https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-breast-cancer/early-breast-cancer
http://www.ncbi.nlm.nih.gov/pubmed/38101773?tool=bestpractice.com
[144]Expert Panel on Breast Imaging, McDonald ES, Scheel JR, et al. ACR appropriateness criteria: imaging of invasive breast cancer. J Am Coll Radiol. 2024 Jun;21(6s):S168-202.
https://www.jacr.org/article/S1546-1440(24)00262-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38823943?tool=bestpractice.com
Biopsy
Biopsy is required for definitive diagnosis. An image-guided core biopsy is usually the preferred method of diagnosis because it enables differentiation between pre-invasive and invasive disease, is less likely to be associated with inadequate sampling, and enables assessment of receptor status.[Figure caption and citation for the preceding image starts]: Inflammatory breast carcinoma showing dermal lymphatic invasion by tumour cellsFrom the collection of Dr Massimo Cristofanilli; used with permission [Citation ends].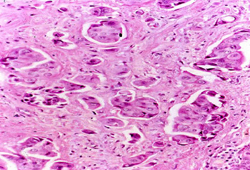
Excisional biopsy may be indicated if core needle biopsy cannot be performed; or results are indeterminate, or are benign and discordant with imaging; or where larger tissue samples are needed. However, it is associated with poorer cosmesis and is more invasive than needle biopsy.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
Fine needle aspiration (FNA) is useful in obtaining a rapid diagnosis of breast malignancy, and it may be the only test required for diagnosis when plans for immediate surgery are already in place. Sensitivity and specificity of FNA are reported to be 98% and 97%, respectively, when performed by experienced clinicians.[145]Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001 Aug 25;93(4):263-8.
https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/cncr.9040
http://www.ncbi.nlm.nih.gov/pubmed/11507700?tool=bestpractice.com
However, diagnostic accuracy with FNA is likely to decline if performed by less experienced clinicians.
Hormone-receptor and human epidermal growth factor receptor 2 (HER2) testing
If a diagnosis of invasive breast cancer is made, the oestrogen receptor (OR), progesterone receptor (PR), and HER2 receptor status of the cancer should be determined to aid treatment and prognostication.[146]Allison KH, Hammond ME, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020 Apr 20;38(12):1346-66.
https://ascopubs.org/doi/full/10.1200/JCO.19.02309
http://www.ncbi.nlm.nih.gov/pubmed/31928404?tool=bestpractice.com
[147]Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists guideline update. J Clin Oncol. 2023 Aug 1;41(22):3867-72.
https://ascopubs.org/doi/10.1200/JCO.22.02864
http://www.ncbi.nlm.nih.gov/pubmed/37284804?tool=bestpractice.com
OR and PR status is assayed using immunohistochemistry (IHC). The American Society of Clinical Oncology (ASCO) and the College of American Pathologists recommend that OR and PR assays should be considered positive if there are at least 1% positive tumour nuclei in the initial biopsy sample.[146]Allison KH, Hammond ME, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020 Apr 20;38(12):1346-66.
https://ascopubs.org/doi/full/10.1200/JCO.19.02309
http://www.ncbi.nlm.nih.gov/pubmed/31928404?tool=bestpractice.com
Women who are OR-borderline are managed in the same way as women who are OR-positive.
Patients diagnosed with breast cancer (early-stage or metastatic disease) should have at least one tumour sample tested for HER2 expression. A HER2 test includes testing for HER2 protein expression (IHC assay) or HER2 gene amplification by in situ hybridisation (ISH).[147]Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists guideline update. J Clin Oncol. 2023 Aug 1;41(22):3867-72.
https://ascopubs.org/doi/10.1200/JCO.22.02864
http://www.ncbi.nlm.nih.gov/pubmed/37284804?tool=bestpractice.com
IHC scoring ranges from 0 to 3+ as determined by intensity of staining, and percentage (>10%) of contiguous and homogeneous positive tumour cells. HER2 status can be classified as follows, based on the IHC score:[147]Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists guideline update. J Clin Oncol. 2023 Aug 1;41(22):3867-72.
https://ascopubs.org/doi/10.1200/JCO.22.02864
http://www.ncbi.nlm.nih.gov/pubmed/37284804?tool=bestpractice.com
HER2 negative: IHC score 0 or 1+
Equivocal: IHC score 2+ (requires reflex testing with ISH assay)
HER2 positive: IHC score 3+.
Single-probe ISH assays measure the average HER2 copy number (signals/cell); dual-probe ISH assay measures the HER2/CEP17 ratio.[147]Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists guideline update. J Clin Oncol. 2023 Aug 1;41(22):3867-72.
https://ascopubs.org/doi/10.1200/JCO.22.02864
http://www.ncbi.nlm.nih.gov/pubmed/37284804?tool=bestpractice.com
The single-probe approach is not preferentially recommended; if used, cases with average HER2 copy number ≥4.0 and <6.0 signals/cell should base final results on concurrent IHC and if 2+ reflexed to dual-probe ISH testing.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
Assuming no apparent histopathological discordance observed by the pathologist, HER2 status can be classified as follows, based on concurrent IHC and ISH results:[147]Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists guideline update. J Clin Oncol. 2023 Aug 1;41(22):3867-72.
https://ascopubs.org/doi/10.1200/JCO.22.02864
http://www.ncbi.nlm.nih.gov/pubmed/37284804?tool=bestpractice.com
HER2 negative:
HER2/CEP17 ratio <2.0 AND average HER2 copy number <4.0 signals/cell (concurrent IHC result not required)
HER2/CEP17 ratio ≥2.0 AND average HER2 copy number <4.0 signals/cell and concurrent IHC score 0, 1+, or 2+
HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥6.0 signals/cell and concurrent IHC score 0 or 1+
HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥4.0 and <6.0 signals/cell and concurrent IHC score 0, 1+, or 2+
HER2 positive:
HER2/CEP17 ratio ≥2.0 AND average HER2 copy number <4.0 signals/cell and concurrent IHC score 3+
HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥6.0 signals/cell and concurrent IHC score 2+ or 3+
HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥4.0 and <6.0 signals/cell and concurrent IHC score 3+
HER2/CEP17 ratio ≥2.0 AND average HER2 copy number ≥4.0 signals/cell (concurrent IHC result not required).
Genetic testing
Performed to detect germline mutations associated with increased cancer risk. It is estimated that 5% to 10% of breast cancers are linked to inherited genetic mutations.[12]Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001 Oct 27;358(9291):1389-99.
http://www.ncbi.nlm.nih.gov/pubmed/11705483?tool=bestpractice.com
[13]Claus EB, Schildkraut JM, Thompson WD, et al. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996 Jun 1;77(11):2318-24.
http://www.ncbi.nlm.nih.gov/pubmed/8635102?tool=bestpractice.com
[14]Honrado E, Benítez J, Palacios J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod Pathol. 2005 Oct;18(10):1305-20.
https://www.nature.com/articles/3800453
http://www.ncbi.nlm.nih.gov/pubmed/15933754?tool=bestpractice.com
BRCA1 and BRCA2 mutations are the most common inherited genetic mutations found in breast cancer.[14]Honrado E, Benítez J, Palacios J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod Pathol. 2005 Oct;18(10):1305-20.
https://www.nature.com/articles/3800453
http://www.ncbi.nlm.nih.gov/pubmed/15933754?tool=bestpractice.com
[15]Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010 May;12(5):245-59.
http://www.ncbi.nlm.nih.gov/pubmed/20216074?tool=bestpractice.com
[16]Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017 Jun 20;317(23):2402-16.
https://jamanetwork.com/journals/jama/fullarticle/2632503
http://www.ncbi.nlm.nih.gov/pubmed/28632866?tool=bestpractice.com
[41]Kurian AW, Ward KC, Howlader N, et al. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J Clin Oncol. 2019 May 20;37(15):1305-15.
https://ascopubs.org/doi/10.1200/JCO.18.01854
http://www.ncbi.nlm.nih.gov/pubmed/30964716?tool=bestpractice.com
Genetic testing can inform prognosis, and aid in systemic therapy and surgical decision-making (e.g., adjuvant olaparib treatment for high-risk, HER2-negative breast cancer; risk-reducing surgery).[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
Genetic counselling and testing for high-penetrance breast cancer susceptibility genes is recommended for certain patients at diagnosis, based on personal or family history, ancestry, diagnosis at an early age, or eligibility for olaparib therapy.[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
[148]Bedrosian I, Somerfield MR, Achatz MI, et al. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology guideline. J Clin Oncol. 2024 Feb 10;42(5):584-604.
https://ascopubs.org/doi/10.1200/JCO.23.02225?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/38175972?tool=bestpractice.com
All male patients with breast cancer diagnosed at any age should have genetic testing.[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
[148]Bedrosian I, Somerfield MR, Achatz MI, et al. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology guideline. J Clin Oncol. 2024 Feb 10;42(5):584-604.
https://ascopubs.org/doi/10.1200/JCO.23.02225?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/38175972?tool=bestpractice.com
National Comprehensive Cancer Network (NCCN) guidelines recommend genetic counselling and testing for high-penetrance breast cancer susceptibility genes (e.g., BRCA1, BRCA2, CDH1, PALB2, PTEN, STK11, and TP53) at diagnosis for the following patients:[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
Diagnosed at aged ≤50 years
With Ashkenazi Jewish ancestry and diagnosed at any age
Males diagnosed at any age
With triple-negative breast cancer, or multiple primary (synchronous or metachronous) breast cancers, or lobular breast cancer (with a personal or family history of diffuse gastric cancer) diagnosed at any age
Candidate for adjuvant olaparib therapy
With any blood relative with a known pathogenic/likely pathogenic variant in a cancer susceptibility gene
With a strong family history, including:
≥1 close blood relative diagnosed with breast cancer at aged ≤50 years, or with male breast cancer, ovarian or pancreatic cancer, or prostate cancer (with metastatic, or high- or very high-risk group) at any age; or
≥3 diagnoses of breast and/or prostate cancer on the same side of the family (including the patient being assessed).
The American Society of Clinical Oncology (ASCO) found that expanding the NCCN age criteria to include all women ≤65 years improved the sensitivity of the criteria (to 98% for BRCA1 or BRCA2). ASCO recommends germline testing for BRCA1 and BRCA2 mutations at diagnosis in the following patients:[148]Bedrosian I, Somerfield MR, Achatz MI, et al. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology guideline. J Clin Oncol. 2024 Feb 10;42(5):584-604.
https://ascopubs.org/doi/10.1200/JCO.23.02225?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/38175972?tool=bestpractice.com
All patients diagnosed with breast cancer aged ≤65 years
Select patients >65 years diagnosed with breast cancer, based on personal or family history, ancestry, eligibility for olaparib therapy.
ASCO guidelines recommend individualised testing for additional high-penetrance genes (e.g., CDH1, PALB2, PTEN, STK11, and TP53) based on personal or family history.[148]Bedrosian I, Somerfield MR, Achatz MI, et al. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology guideline. J Clin Oncol. 2024 Feb 10;42(5):584-604.
https://ascopubs.org/doi/10.1200/JCO.23.02225?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/38175972?tool=bestpractice.com
Genetic testing for a specific pathogenic variant can be carried out, if known; germline multigene panel testing is recommended if the variant is unknown.[18]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic [internet publication].
https://www.nccn.org/guidelines/category_2
Selection of the specific multigene panel should take into account the patient's personal and family history.[148]Bedrosian I, Somerfield MR, Achatz MI, et al. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology guideline. J Clin Oncol. 2024 Feb 10;42(5):584-604.
https://ascopubs.org/doi/10.1200/JCO.23.02225?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/38175972?tool=bestpractice.com
Assessing risk of recurrence
Gene expression assays may be used for prognostication and to guide decisions on adjuvant chemotherapy.[149]Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015 Nov 19;373(21):2005-14.
https://www.nejm.org/doi/full/10.1056/NEJMoa1510764
http://www.ncbi.nlm.nih.gov/pubmed/26412349?tool=bestpractice.com
[150]Goncalves R, Bose R. Using multigene tests to select treatment for early-stage breast cancer. J Natl Compr Canc Netw. 2013 Feb 1;11(2):174-82.
https://www.jnccn.org/content/11/2/174.long
http://www.ncbi.nlm.nih.gov/pubmed/23411384?tool=bestpractice.com
[151]Harbeck N, Sotlar K, Wuerstlein R, et al. Molecular and protein markers for clinical decision making in breast cancer: today and tomorrow. Cancer Treat Rev. 2014 Apr;40(3):434-44.
http://www.ncbi.nlm.nih.gov/pubmed/24138841?tool=bestpractice.com
[152]Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019 Jun 20;380(25):2395-405.
https://www.nejm.org/doi/10.1056/NEJMoa1904819
http://www.ncbi.nlm.nih.gov/pubmed/31157962?tool=bestpractice.com
[153]Andre F, Ismaila N, Allison KH, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022 Jun 1;40(16):1816-37.
https://ascopubs.org/doi/10.1200/JCO.22.00069
http://www.ncbi.nlm.nih.gov/pubmed/35439025?tool=bestpractice.com
Oncotype DX® is the preferred assay to determine whether the addition of chemotherapy to endocrine therapy would be of benefit in patients with HR-positive, HER2-negative disease who are node-negative or post-menopausal with node-positive disease (1-3 positive nodes).[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
[153]Andre F, Ismaila N, Allison KH, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022 Jun 1;40(16):1816-37.
https://ascopubs.org/doi/10.1200/JCO.22.00069
http://www.ncbi.nlm.nih.gov/pubmed/35439025?tool=bestpractice.com
[154]National Institute for Health and Care Excellence. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. Dec 2018 [internet publication]
https://www.nice.org.uk/guidance/dg34
Oncotype DX® is a reverse transcription polymerase chain reaction-based multigene assay that evaluates the expression of 21 genes within the patient's paraffin-embedded tumour slides.[155]Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004 Dec 30;351(27):2817-26.
https://www.nejm.org/doi/full/10.1056/NEJMoa041588
http://www.ncbi.nlm.nih.gov/pubmed/15591335?tool=bestpractice.com
Based on this expression, a low (≤10), intermediate (11-25), or high (26-100) recurrence score can be calculated. The recurrence score can aid decision-making on whether a patient with hormone receptor-positive breast cancer who is node-negative or positive for 1-3 nodes would benefit from adjuvant chemotherapy, or if adjuvant endocrine therapy alone would be sufficient.[149]Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015 Nov 19;373(21):2005-14.
https://www.nejm.org/doi/full/10.1056/NEJMoa1510764
http://www.ncbi.nlm.nih.gov/pubmed/26412349?tool=bestpractice.com
[156]Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018 Jul 12;379(2):111-21.
https://www.nejm.org/doi/10.1056/NEJMoa1804710
http://www.ncbi.nlm.nih.gov/pubmed/29860917?tool=bestpractice.com
[157]Sparano JA, Gray RJ, Makower DF, et al. Clinical outcomes in early breast cancer with a high 21-gene recurrence score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: a secondary analysis of the TAILORx randomized clinical trial. JAMA Oncol. 2019 Sep 30;6(3):367-74.
https://jamanetwork.com/journals/jamaoncology/fullarticle/2752332
http://www.ncbi.nlm.nih.gov/pubmed/31566680?tool=bestpractice.com
[158]Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021 Dec 16;385(25):2336-47.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9096864
http://www.ncbi.nlm.nih.gov/pubmed/34914339?tool=bestpractice.com
Pre-menopausal patients with 1-3 positive nodes benefit from chemotherapy regardless of genomic assay result. The clinical utility of assays in node-positive disease with ≥4 nodes is unknown.[153]Andre F, Ismaila N, Allison KH, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022 Jun 1;40(16):1816-37.
https://ascopubs.org/doi/10.1200/JCO.22.00069
http://www.ncbi.nlm.nih.gov/pubmed/35439025?tool=bestpractice.com
Other assays, such as Mammaprint®, Breast Cancer Index (BCI), Prosigna®, and EndoPredict®, may be used to provide prognostic information in post-menopausal women or women aged >50 years who are node negative. Mammaprint® and EndoPredict® may also be used for post-menopausal women or women aged >50 years with 1-3 positive nodes. However, the ability of these assays to predict therapeutic benefit is less certain.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
[153]Andre F, Ismaila N, Allison KH, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022 Jun 1;40(16):1816-37.
https://ascopubs.org/doi/10.1200/JCO.22.00069
http://www.ncbi.nlm.nih.gov/pubmed/35439025?tool=bestpractice.com
Blood tests
Blood tests are not generally recommended as part of staging and preoperative work-up.
A full blood count, a comprehensive metabolic panel, liver function tests, and an alkaline phosphatase test should be considered only if the patient is a candidate for preoperative or adjuvant systemic therapy.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx
Patients with a clinical/pathological diagnosis of inflammatory breast cancer without distant metastasis should have a full blood count and platelet count.[117]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].
https://www.nccn.org/professionals/physician_gls/default.aspx


