Aetiology
The gene for G6PD is located at the end of the long arm of the X chromosome. The deficiency is thus seen in hemizygous males (who have a mutation on their sole X chromosome) and in homozygous females (who have a mutation on each of their X chromosomes; very rarely seen in most X-linked diseases, but frequent in populations where G6PD deficiency is common [e.g., Africa]).
High prevalence of G6PD mutations may be explained by the protective benefit that G6PD-deficient alleles confer against the malarial parasite Plasmodium falciparum (similar to that seen in sickle cell disease and the thalassaemias). The exact nature of the protective mechanism is uncertain. Evidence to support this is derived from population studies that show the global distribution of G6PD-deficient alleles is similar to the worldwide distribution of malaria.[4] In addition, a clinical study has shown that the presence of specific mutations (e.g., the G6PD A- allele) reduces the risk of severe Plasmodium falciparum malaria by 46% to 58%.[9] In vitro studies have shown that in heterozygous females, parasitisation of normal red cells by this microbe is 2 to 80 times greater than in G6PD-deficient cells.[4]
Heterozygous females are genetic mosaics: half of their cells are normal; the other half are completely deficient in G6PD. They are usually only very mildly symptomatic, but severe acute haemolysis sometimes occurs. Mutations in the gene lead to amino acid substitutions (or, rarely, small deletions) that alter the 3-dimensional structure of the enzyme and cause reduced enzyme activity. Approximately 160 mutations have been identified, far fewer than the number of previously described biochemical variants. Many of these variants appear to have arisen independently in different parts of the world and are now known to be identical at the molecular level.[10] Structure-function relationships have been defined with clinical correlation. Therefore, the mutations that underlie class I variants result in a much more unstable enzyme and are associated with markedly reduced activity, whereas the mutations leading to class III and IV variants are associated with a greater conservation of enzyme activity.[11][12]
Common specific genetic variants include:
G6PD Mediterranean: a class II variant affecting people from European and Arabic countries around the Mediterranean (or their descendants)
G6PD A-: a class III variant affecting people from sub-Saharan Africa (or their descendants)
G6PD Mahidol (class III) and Union (II): commonly affect Asian populations.
Pathophysiology
G6PD is required by all cells to protect them from damage by oxidation. It catalyses the first step in the pentose phosphate pathway, whereby glucose-6-phosphate is oxidised to 6-phosphogluconate. This reaction is linked to the reduction of NADP (nicotinamide adenine dinucleotide phosphate) to NADPH (reduced nicotinamide adenine dinucleotide phosphate), which is used to generate reduced glutathione.
[Figure caption and citation for the preceding image starts]: Role of glucose-6-phosphate dehydrogenase in the pentose phosphate pathway resulting in the generation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) and reduced glutathione (GSH), products required to protect the red blood cell from oxidative stressAdapted from Haematology at a Glance, Mehta AB and Hoffbrand AV, 3rd Edition Wiley Blackwell 2007; used with permission [Citation ends].
For the red cell, this is the sole source of protection against oxidative damage. Red blood cells are constantly challenged by oxidants in the form of free radicals generated by the conversion of oxyhaemoglobin to deoxyhaemoglobin and by peroxides generated by phagocytosing granulocytes. Normal red cells can increase generation of NADPH in response to oxidative stress; this capacity is impaired in patients with G6PD deficiency. Failure to withstand oxidative stress damages sulphydryl groups in haemoglobin and the red cell membrane and causes haemolysis. Cells in other tissues and organs have alternate pathways for the generation of NADPH and can thus withstand such oxidative stress. In contrast, red cells are metabolically extremely simple; they lack a nucleus and mitochondria, cannot carry out protein synthesis, and exclusively metabolise glucose for ATP production. The activity of all red cell enzymes, including G6PD, is highest in young red cells (reticulocytes), and progressively declines as the cell ages.
Following a haemolytic episode, the rate of red cell production is accelerated and the increased proportion of young red cells with higher levels of G6PD limits further red cell lysis. Haemolytic episodes are therefore usually self-limiting in those with moderately deficient (class III) variants (for example, G6PD A-) but may result in more severe and progressive anaemia in patients with severely deficient (class II) variants.
Complete lack of the enzyme is incompatible with life. Rarely, male patients with class I variants have profoundly low G6PD activity, such that the red cells are constantly undergoing lysis (chronic non-spherocytic haemolytic anaemia). Almost all deficient people, however, have one of the polymorphic enzyme variants (e.g., G6PD Mediterranean, class II, or G6PD A-, class III) that bestow sufficient residual activity to maintain the person in an asymptomatic state under non-stressed conditions. However, when provoked by oxidative stress (e.g., ingestion of certain drugs, exposure to certain chemicals or glycosides in broad beans, and infection), red cell lysis occurs.
Some drugs are known to pose an oxidative challenge to the red cells and to induce haemolytic anaemia in patients with G6PD deficiency. For some drugs (e.g., dapsone, primaquine, sulphonamides), the association is very clear and consistent, and haemolysis will be observed in virtually all patients, irrespective of residual enzyme activity.[2][12][13][14] For others (for example, aspirin) the clinical association is less consistent and reflects an interplay of inherited (e.g., residual enzyme activity, pharmacokinetics) and acquired (e.g., dose, absorption and drug metabolism, co-existing infection) factors.[2][12] The importance of primaquine-induced haemolysis in Latin America has been highlighted.[15] One review emphasised that many compounds may have been wrongly cited as causing haemolysis because they were administered to individuals who were suffering infection-related haemolysis.[16] This review highlighted the haemolytic potential of only 7 currently used medications: dapsone, methylene blue, nitrofurantoin, phenazopyridine, primaquine, rasburicase, and toluidine blue.[Figure caption and citation for the preceding image starts]: Drugs and agents that are known to cause haemolysis in people with G6PD deficiencyCreated by Professor Atul B. Mehta [Citation ends].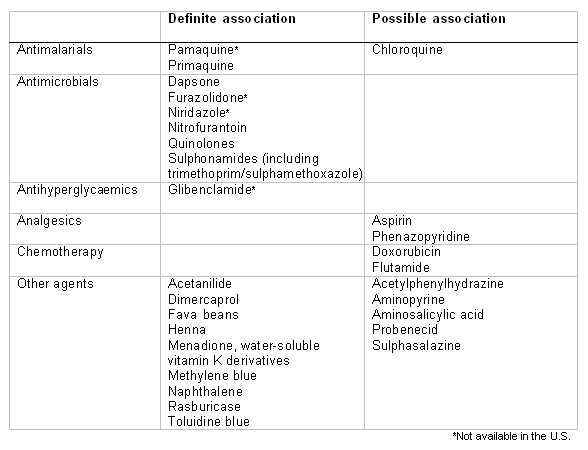
During the neonatal period, hyperbilirubinaemia (physiological jaundice) is frequently exaggerated in G6PD-deficient patients because of increased haem turnover from red cell lysis and the impaired ability to conjugate and clear bilirubin. This clinical picture is more common with the more severe G6PD variants, and hyperbilirubinaemia is more severe when the person also has an inherited deficiency of the enzymes required to conjugate bilirubin.[17][18][19][20][21]
Abnormalities in bilirubin metabolism in patients with G6PD deficiency are believed to contribute to the increased prevalence of cholelithiasis in these patients.[22][23][24]
Classification
G6PD deficiency WHO working group[1]
Deficient activity of the enzyme usually results from structural mutations that reduce the stability of the enzyme. More than 400 variant enzymes have been characterised according to biochemical and physicochemical properties. These variants have been classified into 5 groups based on enzyme activity and clinical manifestations:
Class I: severely deficient, associated with chronic non-spherocytic haemolytic anaemia
Class II: severely deficient (1%-10% residual activity), associated with acute haemolytic anaemia
Class III: moderately deficient (10%-60% residual activity)
Class IV: normal activity (60%-150%)
Class V: increased activity (>150%).
Use of this content is subject to our disclaimer